Materials for Lithium-ion batteries
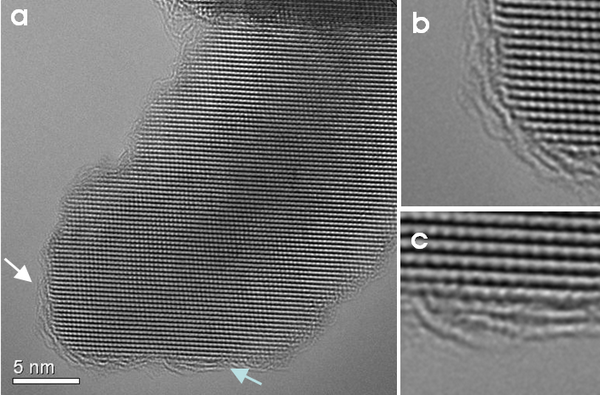
To understand the structural mechanisms of the Li-ion insertion and exsertion mechanism is of particular relevance for the optimisation of Lithium-ion batteries. During the charging/discharging of the battery Li-ions diffuse from the cathode to the anode and vice-versa. The electrodes consist of nanomaterials embedded into a conductive binder. These nanomaterials, typically oxides like LiFePO4 or TiO2, can intercalate and release the Li-ions. Using TEM the structural changes in these nanomaterials are studied in order to understand why the electrochemical deactivation process is faster in some materials than in others. Technological aspects like the influence of nanoparticle coatings on the electrochemical performance are also studied. Figure 1 shows a nanoparticle that is coated by graphitic layers.
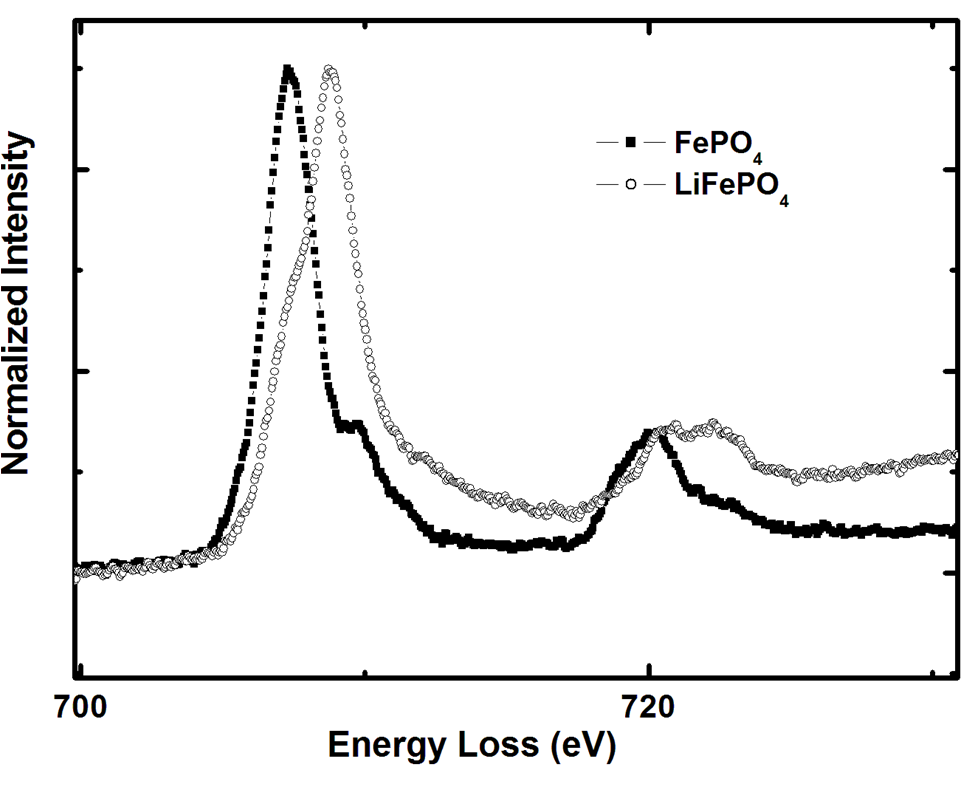
Another important task for TEM investigations is to follow the changes in the electric and electronic properties of charged and discharged material. Electron Energy Loss Spectrometry (EELS) is used in order to determine e.g. the Li-ion content of the crystallite or the dielectric constant of the material at different stages of charging (see Figure 2).The repetitive insertion and excerption of the ions into the crystal causes dilatation and contraction of the structure and thus, over time, the crystal structure is damaged. Using TEM the structural mechanisms of the damage process can be studied on the atomic scale. In some materials the Li-ions can only diffuse along particular crystallographic directions. In this case only TEM investigations can reveal why strongly anisotropic crystals of some materials show an unexpected high electrochemical activity and crystals with more isotropic morphology of the same material do not.
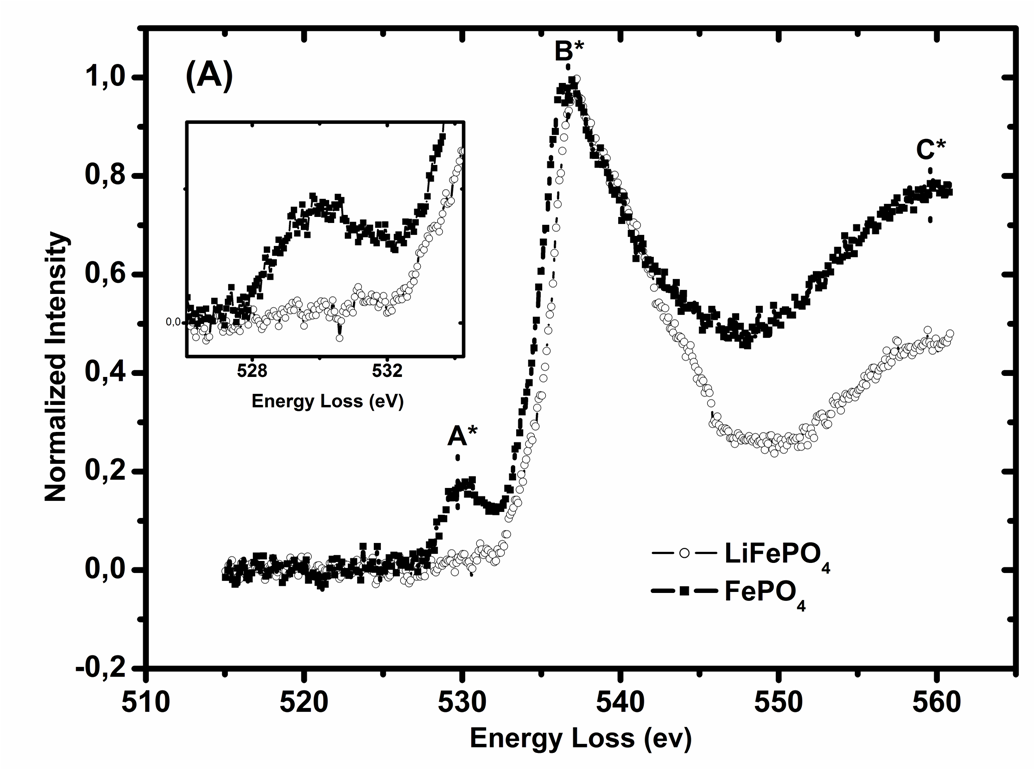
Another effect under investigation is the charge compensation processes within the batteries. The core energy loss region of an EELS spectrum provides information regarding the excitation of the electrons at the from the deep lying core states. These excitations provide information regarding the partial density of unoccupied states. The differences in the pre-edge peak of the O K edge indicate the formation of holes in the Oxygen 2p states with delithiation. The charge compensation process involves both Fe and O ions. The oxidation state of the Fe ion changes with delithiation and holes are formed at the O sites.
[1] P. Kubiak, M. Pfanzelt, J. Geserick, U. Hörmann, N. Hüsing, U. Kaiser and M. Wohlfahrt-Mehrens
Electrochemical Evaluation of Rutile TiO2 Nanoparticles as Negative Electrode for Li-ion Batteries
Journal of Power Sources, 194, 1099 – 1104 (2009)
[2] Michael Kinyanjui
Electronic properties and structural properties of Li (1 - chi) FePO 4 (X = 0 , 0.5 ,1)
Ph.D. thesis (2010)