Head
Prof. Cornelia Brunner
Tel: 0731 500 59714
Core Facility Manager
Dr. Sybille Kempe
Tel: 0731 500 59715
The ULMTeC Core Unit Immune Monitoring of solid tumors offers a service platform of the medical faculty and the University Medical Center Ulm.
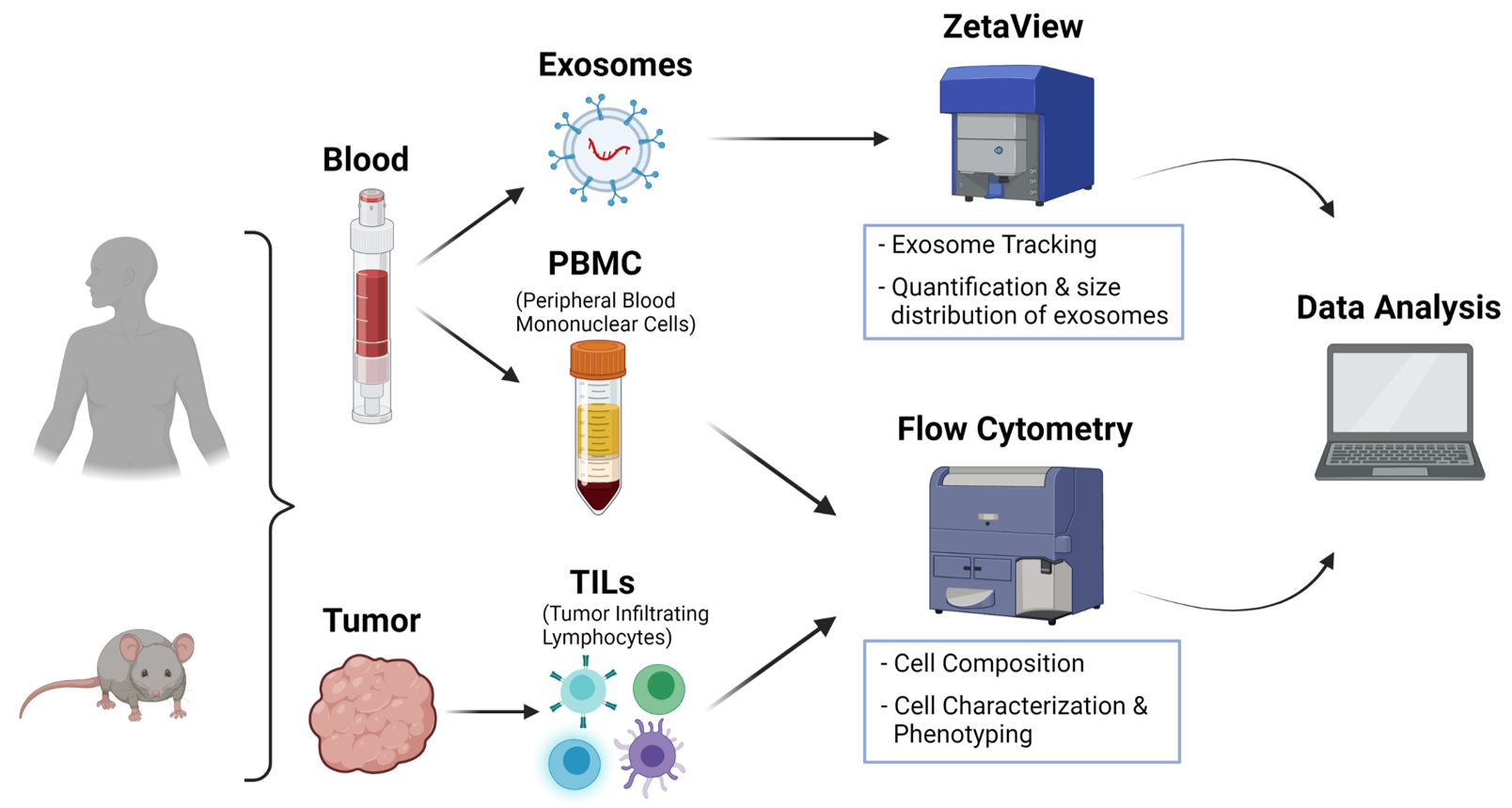
Our goal is to provide an optimal full-service in the field of immune monitoring of human and murine solid tumors to all members of the University Medical Center and Ulm University as well as to external industrial and academic partners. Our full-service extends from sample preparation to the staining of samples using standardized panels for immune phenotyping as well as data analysis and interpretation. Human (e.g. tumors, PBMCs) as well as murine (e.g. tumors, blood, lymphatic organs) tissue can be prepared and analyzed.
QuickLinks
Services
The services provided include:
- Initial project planing
- Sample preparation (human/mouse)
- Development and establishment of individual panels
- Data analysis
We also offer exosome isolation and quantification and size distribution of exosomes as well as cell characterisation and phenotyping using standardised staining panels. Furthermore, we offer our service of immune monitoring of immunologically relevant organs for all research areas (e.g. liquid tumours, trauma, ageing, neurodegenerative diseases).
Equipment
Spectral flow cytometer Aurora from Cytek
Technology of spectral cytometry and spectral unmixing allows greater fluorochrome choice, panel flexibility, and easy setup without the need to change filters.
The spectral flow cytometer Aurora provides:
- 4 lasers (blue, red, violet, UV) and 57 detection channels (54 fluorescence channels, FSC, blue laser SSC, violet laser SSC) enable detection over the full emission spectra
- measure single tubes or in 40-tube rack or 96-well plate formats
- high throughput: mode (100 µl/min), 35,000 events per second and throughput 96-well plate: 27 minutes at high throughput mode sampling 7 µl/well
- ‘small particle‘ detector: particles nearing 100 nm in size can be analyzed
- autofluorescence extraction improves resolution
- Ca2+ measurements possible
- volumetric measurement during sample recording enables calculation of counts per µl for any gated population
Particle Metrix ZetaView Tracking Analyzer
In addition, we offer the isolation of exosomes and their subsequent analysis using ZetaView. This technique allows individual tracking and measuring of exosomes and all measurements can be viewed and saved in video format. The device consists of a cell unit, a laser (488 nm), a microscope and a video camera.
- Nanoparticle Tracking Analysis (NTA) Instrument for tracking the Brownian movement of individual visualised nanoparticles in suspension (microvesicles and exosomes)
- Real-time visualisation of Brownian motion and electrophoretic mobility, for measuring size and concentration in the scattered light or fluorescence mode.
- Very short measuring times for the detection and analysis of typically 1000 particles in about 1 minute
- Concentration range: 105 – 109 particles/ml
- Particle size: 10nm - 2000nm (depending on sample and laser selection)