Group Leader
Prof. Dr. Timo Jacob
The combination of electrochemistry with biological systems and questions opens up this exciting field of work. In addition to the functionalization of metallic electrodes with organic or biological materials, the focus is primarily on the development of biosensors and biocatalysts.
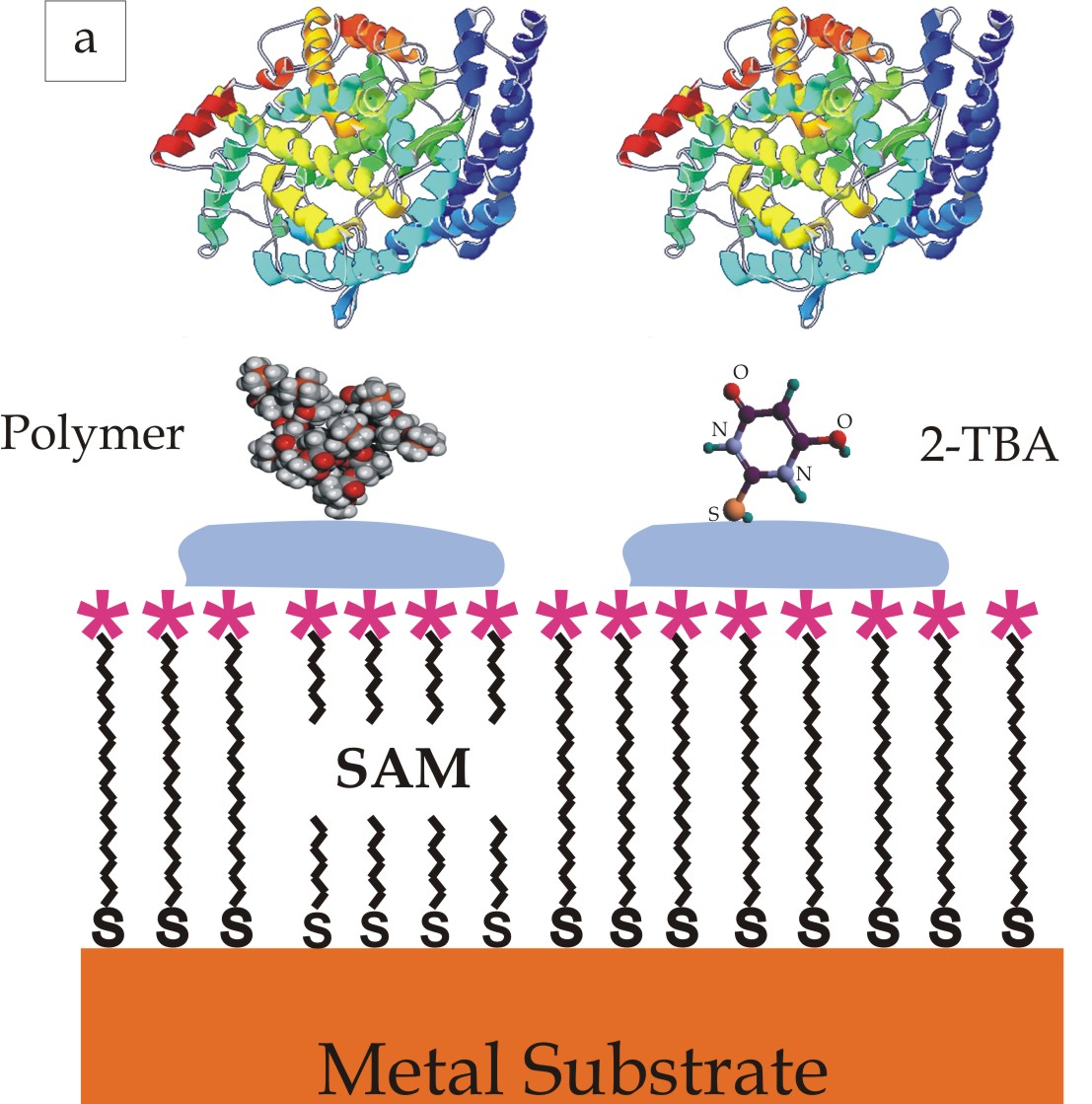
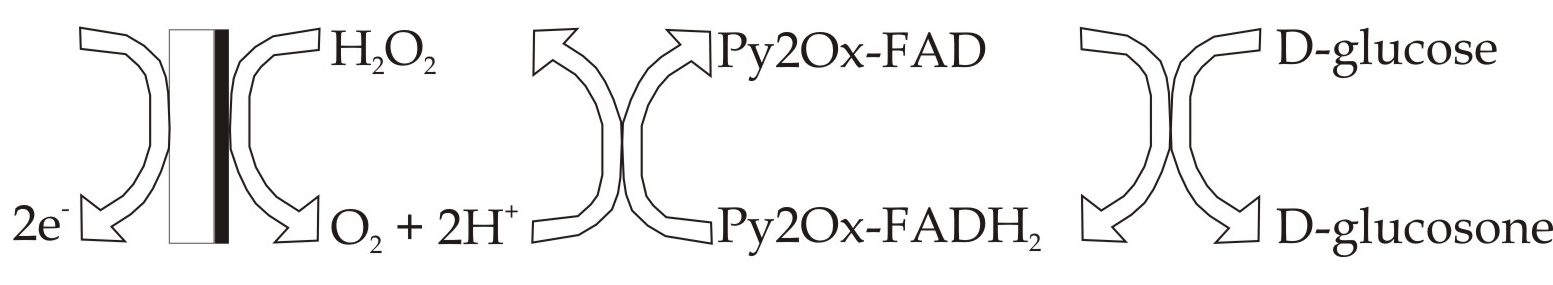
By immobilizing enzymes on conductive surfaces, an interface between artificial and biological systems can be established. This interface enables a direct electrical communication with the enzyme. This circumstance can be used to construct selective biosensors for certain molecules. If such molecules are present, specific redox enzymes catalyze the oxidation or reduction of these molecules. Those redox reactions cause a measurable electric current making the enzymatic activity visible. Figuratively, the system tells us something about the processes taking place. Thus, it delivers information and we receive them.
However, the function of an enzymatic sensor can be reversed by switching the direction of the flow of information. In that case, we provide the system with information by applying a potential and supplying specific molecules. The enzyme is forced to catalyze the desired redox reaction. Consequently, the enzyme electrode can also be utilized for synthesis. Since enzymes are two-way biocatalysts, they enable a broad variety of reactions.
Group Leader
Prof. Dr. Timo Jacob
Electrochemical processes on biomolecules are of interest for a number of applications, such as analytics, diagnostics and fundamental understanding.
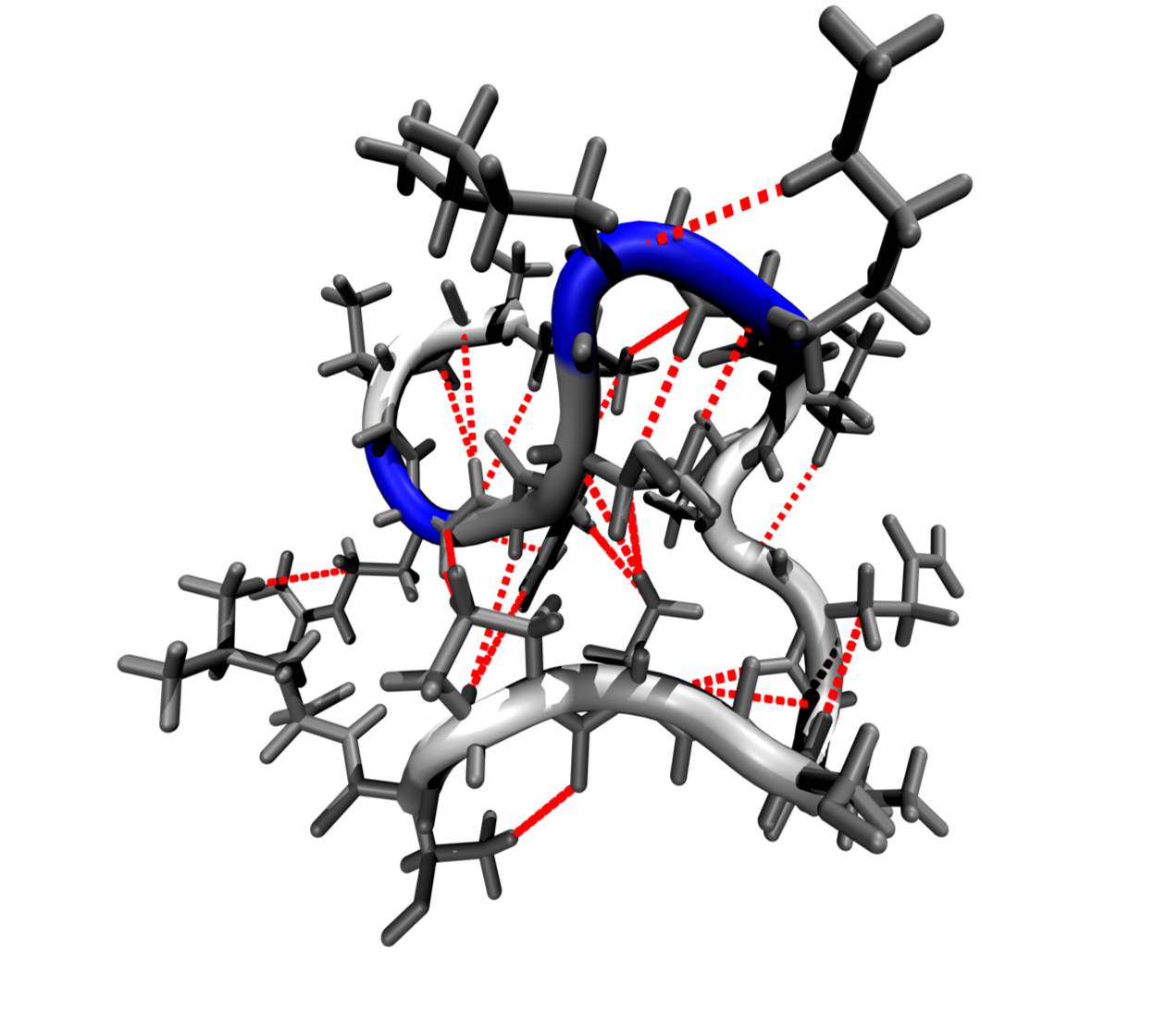
Focusing on DNA systems and peptides our aim is to understand the functionalization of electrodes with organic molecules down to the atomistic scale. Here, theoretical studies are extremely helpful to elucidate the structure of these interfaces and the ongoing processes, information that are often lacking and hardly accessible experimentally.
Thus, by combining ab-initioquantum-mechanical methods with classical and reactive forcefields we are exploring the interaction (and docking) between peptides and also their attachment to electrodes.
As entropy is the strongest driving force for reactions besides energy, we are also developing methods to accurately include these thermodynamic contributions. So far calculating the entropy for a given process was mostly restricted to solid or gas-like systems. To expand the evaluation of entropy contributions to liquid systems as well we adopted the 2PT-method for use with the ReaxFF framework.