Group Leader
Dr. Ludwig Kibler
Dr. Attila Farkas
Dr. Mohammad Al-Shakran
Dr. Johannes Hermann
The focus of this research area currently includes the diverse questions about the interface between electrode and electrolyte, i.e. the area where electrochemical reactions take place. In addition to the structure of the electrode / electrolyte interface, we also examine the processes taking place there, e.g. SEI formation in batteries, degradation mechanisms, catalyst (further) development, process optimization, etc., also the reaction processes in batteries or fuel cells.
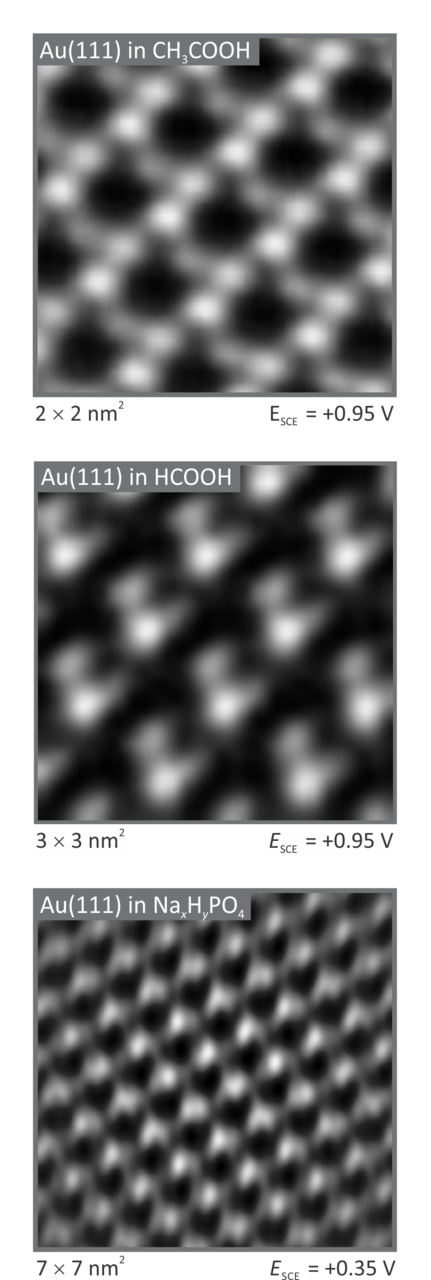
Fundamental understanding and resolving local electrochemical reactions at the metal/electrolyte interface holds the key to enabling transformative advances in energy storage, catalysis and biological applications. Identifying relationships between the microscopic properties of adsorbed reactants and intermediates and the macroscopic kinetic rates of electrochemical reactions could help to create tailor-made surfaces with the microscopic structure required to achieve the desired catalytic properties. In-situ microscopic imaging of an electrode surfaces helps reveal the structural properties of adlayers on surfaces, on an atomic level, in real space, to compare with results from macroscopic techniques. By doing so, it is possible to reveal the specifics of electrochemical phenomena as a function of not only the nature of an electrolyte but also the surface structure of an electrode. A powerful method like in-situ STM can unlock the mysteries of the electrochemical double-layer, where to date often only speculations exist within the scientific community.
Group Leader
Dr. Ludwig Kibler
Dr. Attila Farkas
Dr. Mohammad Al-Shakran
Dr. Johannes Hermann
...will follow shortly
Group Leader