Research Area - Metal Deposition
Electrochemical metal deposition is a classic field of work in electrochemistry. The initial stages and the study of electrocrystallization at the atomic level are of particular interest in basic research. Underpotential deposition enables pseudomorphic monolayers of foreign metals to be obtained on single-crystal electrode surfaces. With these model systems it is possible to better understand the behavior of binary catalysts. Nanostructured surfaces, thin films and alloys can also be obtained by metal deposition. Aqueous solutions or molten salts are mostly used as electrolytes. The deposition of precious metals is itself a subject of research, the structures created are also used for basic investigations in electrocatalytic and battery research.
Experiment
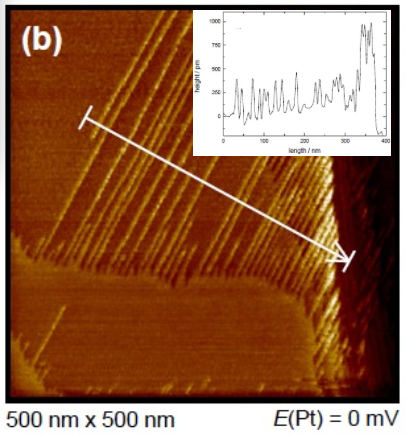
Reactive metals can be separated from ionic liquids. These are solvent-free electrolytes that are liquid at room temperature. The special properties of ionic liquids make them interesting for research but also for industry. Lithium, magnesium or sodium cannot be separated from water solution or only with difficulty. These metals play an important role in the field of energy storage and are being intensively researched for future applications. A basic understanding of the elementary processes is an essential part of the research. Controlling the initial stages of metal deposition (nucleation and growth) can affect the structure of the deposited material. A research focus is therefore the (undesirable) dendritic growth of lithium in battery systems (see figures). In addition to the battery-related topics, copper and silver are also separated from ionic liquids. These metals can also be deposited from aqueous electrolytes. In this way, the influence of the electrolyte and the solvent on elementary processes in metal deposition can be investigated.
Theory
![[Translate to english:] Li-Abscheidung](/fileadmin/website_uni_ulm/nawi.inst.080/images/Forschung/Li-Abscheidung_Theorie.png)
During repeatedly charging and recharging battery systems, the corresponding metal ions are consecutively deposited from or resolved into the electrolyte. However, rather than depositing (or growing) homogeneously as a flat metal foil, mostly non-uniform surface growth occurs, finally leading to morphological changes of the electrode and the interface as well as the formation of needle-like (or dendritic) structures.
Therefore, in tight connection to our experimental investigations our theoretical activities are aiming at studying the underlying mechanisms by which the different metals are deposited on the electrodes and explore potential ways to inhibit inhomogeneous surface growth.
However, the individual processes of metal deposition take place on different time and length scales and consequently cannot be handled by a single theoretical approach without the loss of crucial information. Linking different theoretical methods to a multi-scale approach we are aiming at a detailed picture of the metal deposition and dissolution on the atomic scale.
Group Leader
