The influence of a solid support on the dynamics of a molecular encounter is of fundamental interest to all aspects of surface chemistry and catalysis. An issue of central importance in this respect is the geometrical alignment of the initial collision complex, which determines the passage through the transition state and ultimately the outcome of the desired chemical reaction. The motion involved in this process occurs on the ultrafast timescale of nuclear movement and its understanding is fundamental to the perception of chemical reaction mechanisms on surfaces of, e.g., catalytic materials.
However, in order to be able to unravel the decisive molecular dynamics, the averaging over an ensemble of impact parameters and trajectories originating from variations in the starting geometry has to be minimized.
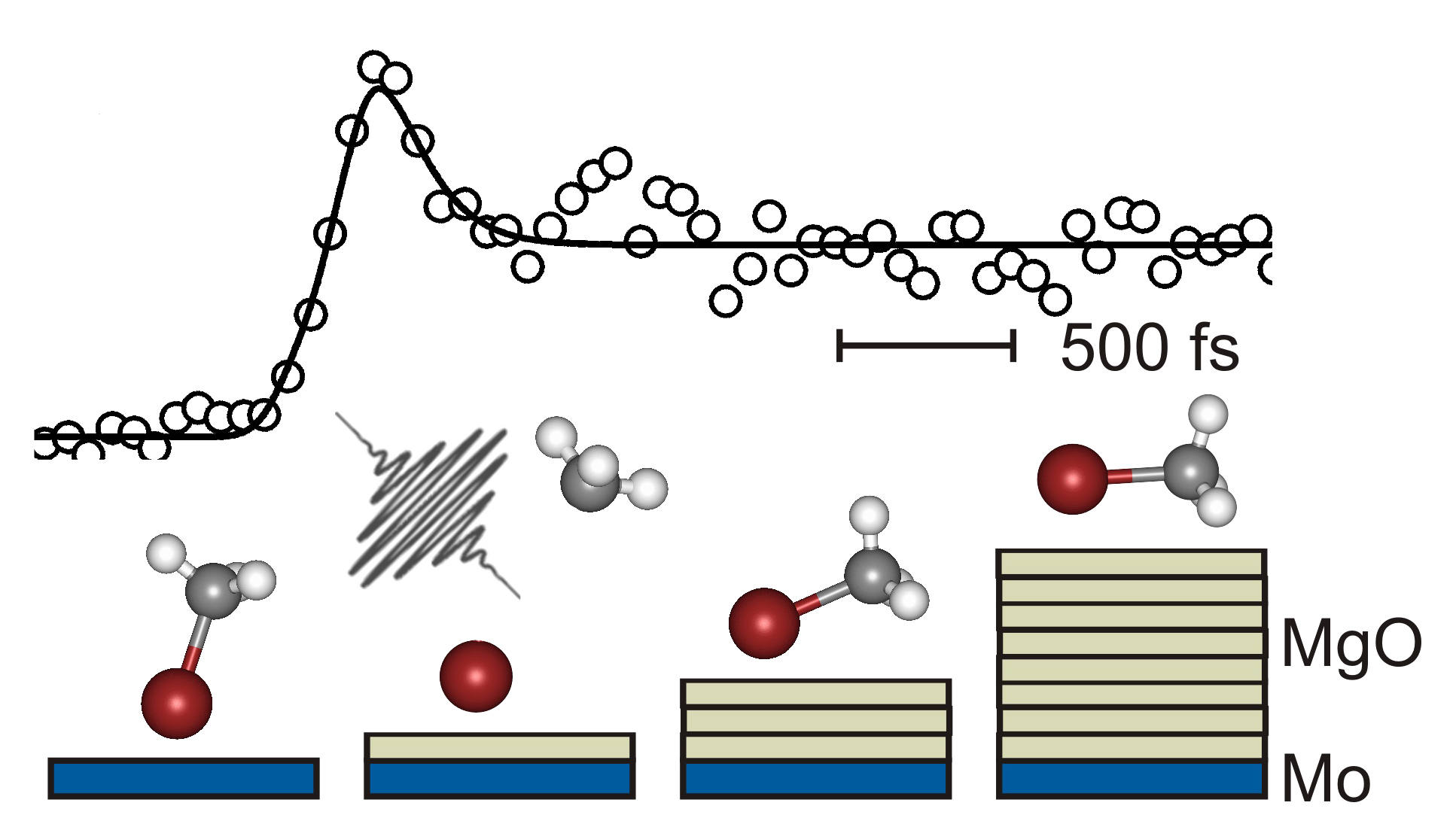
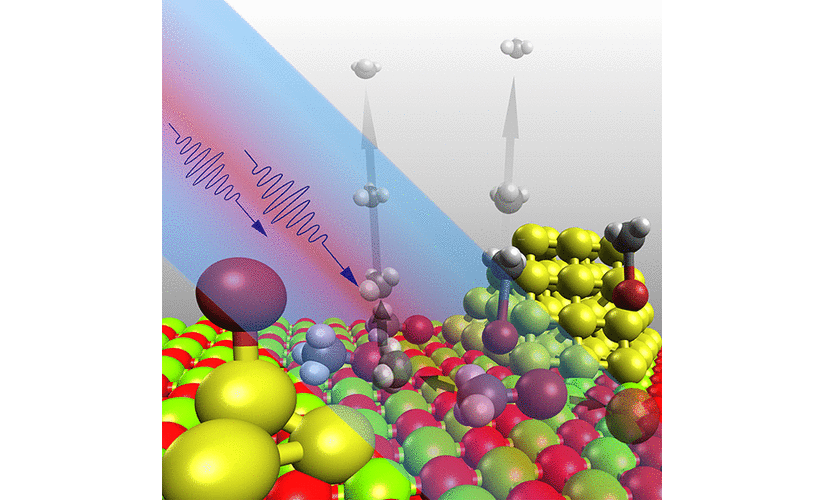
In gas phase experiments this can be realized by employing van der Waals complexes of reactants with defined geometry that are prepared in a supersonic molecular beam. On surfaces a complementary approach has been pioneered by John C. Polanyi which was termed “surface-aligned reaction” and which relies on an ordered adsorbate structure on a solid surface. In our laboratory this idea was realized by a novel experimental approach for direct mass resolved monitoring of surface transition states and products.
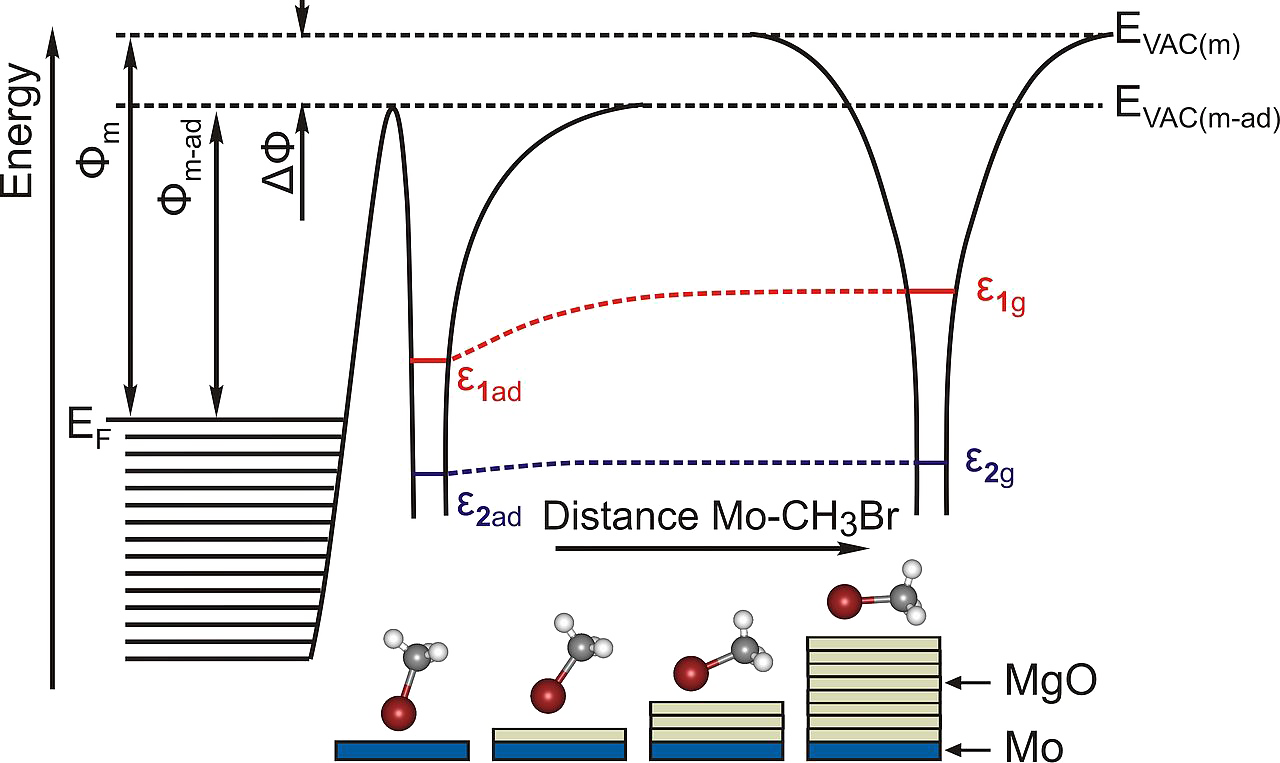
Complementary two photon photoemission spectroscopy experiments reveal the electronic structure of the investigated substrates.
M. E. Vaida, T. M. Bernhardt:
Surface pump-probe femtosecond-laser mass spectrometry: Time-, mass-, and velocity-resolved detection of surface reaction dynamics,
Rev. Sci. Instrum. 81, 104103 (2010).
Z. Ning, J. C. Polanyi:
Surface-aligned reaction,
J. Chem. Phys. 137, 09170 (2012)
M. E. Vaida, T. M. Bernhardt:
Surface-aligned femtochemistry: Molecular reaction dynamics on oxide surfaces,
in “Ultrafast phenomena in molecular sciences”, Ed. R. de Nalda and L. Bañares, Springer Verlag, Berlin (2014), S. 231.