Contact
- Dr. habil. Tamás Röszer
- Fax: +49 (0) 731-50-22629
- E-mail: tamas.roeszer(at)uni-ulm.de
- roszer.tamas(at)med.unideb.hu
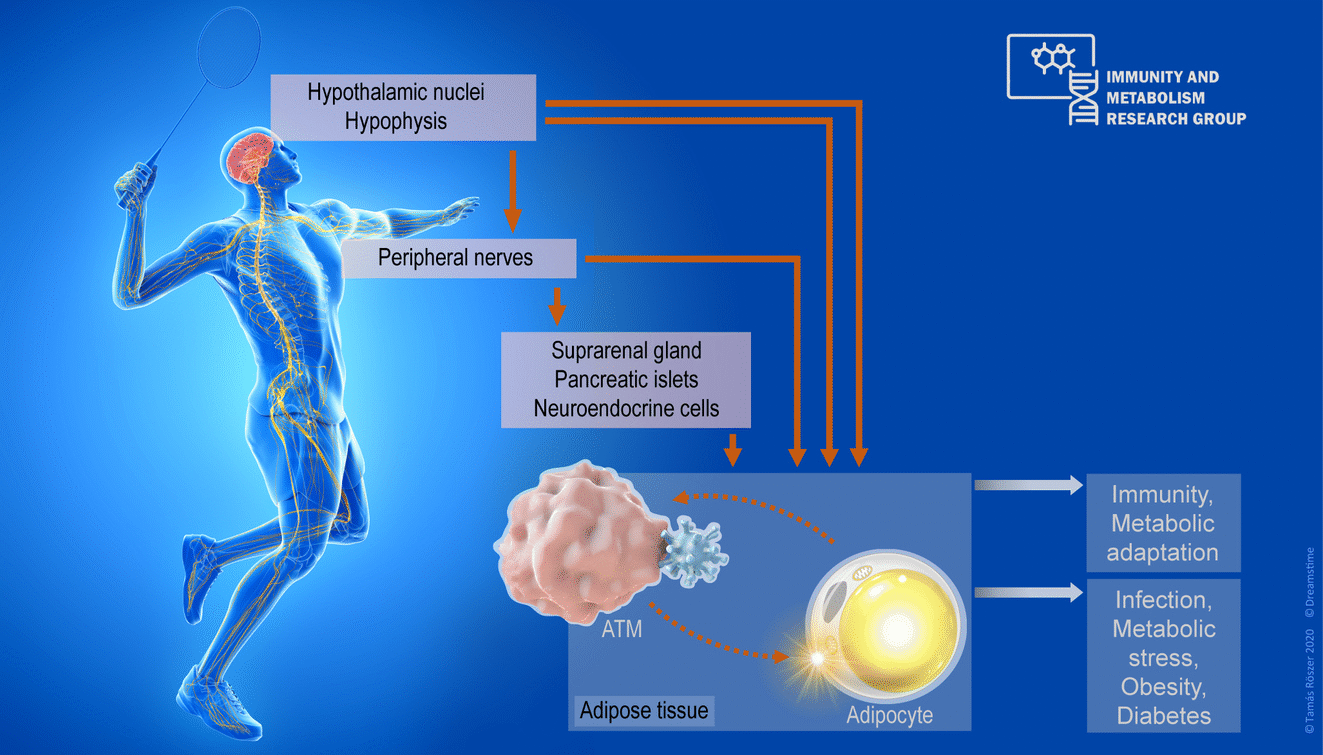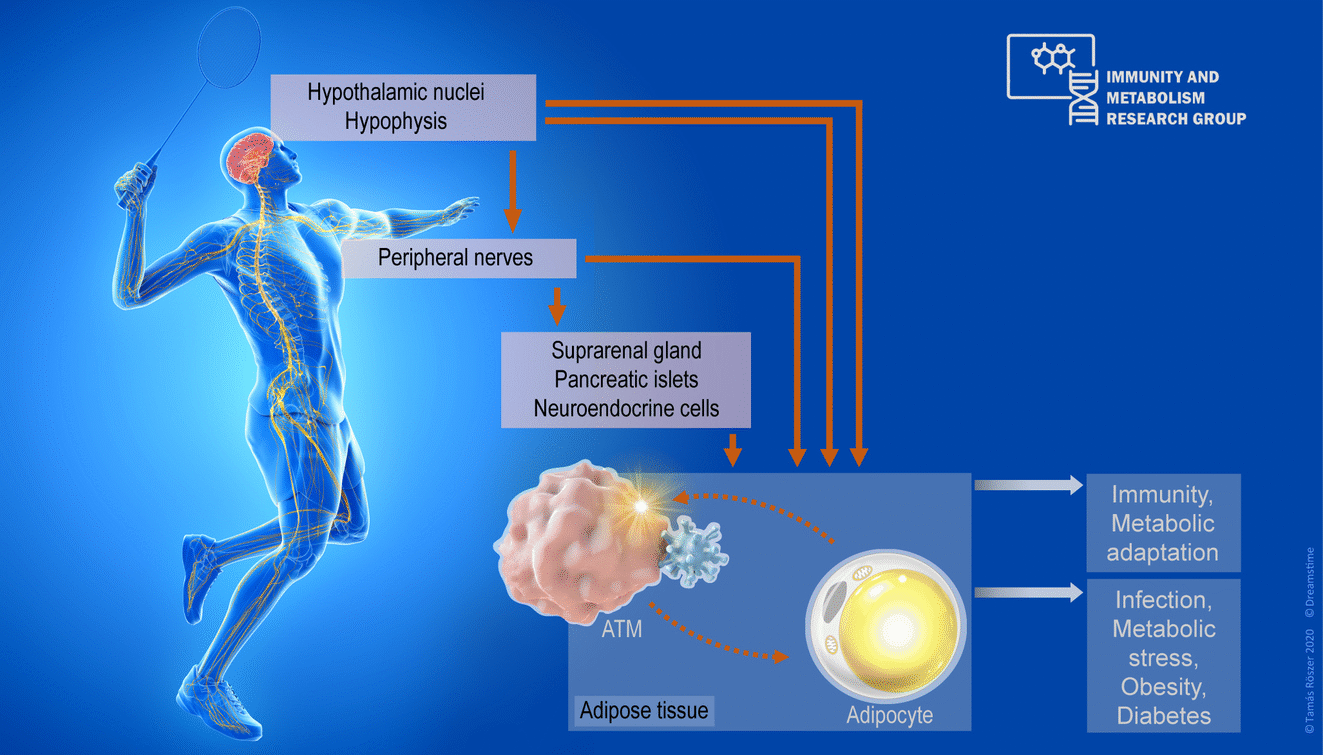
Immune surveillance and metabolism are two principles sustaining life. Immune cells support the organism's growth and metabolism. Dysfunctional interplay between the immune system and metabolism drives the development of metabolic diseases such as insulin resistance, metabolic syndrome and diabetes mellitus. Our research aim is to better understand how the neuroendocrine system governs the interaction between immune cells and metabolic tissues. We use adipose tissue as a model system, in which we have explored non-canonical mechanisms, such as neuropeptide signaling, and breast milk mediated mother-to-child signaling, which control the number and behavior of adipose tissue macrophages (ATMs). ATMS are metabolically important immune cells. We have found that ATMs develop before birth, and ATMs in infancy maintain heat-generating ("fat-burning") beige adipose tissue. Interestingly, neuropeptide signals guide the ability of ATMs to fulfill this task. Infants not fully breast-fed have impaired ATM functions and are more prone to premature loss of their beige adipose tissue, which might lead to the development of obesity and metabolic diseases in later life. Video summaries of our recent research are available on the website of The Journal of Clinical Investigation (NPFF effects on ATMs, Breast-milk lipid signaling and adipose tissue development), in Science and on the webpage of the European Commission CORDIS. Currently, we explore further how the nervous system orchestrates signaling between ATMs and adipocytes to keep the adipose tissue metabolically fit. Our work is supported by the German Research Fund (DFG), the European Foundation for the Study of Diabetes (EFSD), and a PRC Fellowhip Program.
With the global increase of childhood obesity and diabetes, our reserch topic is timely and has societal impact. This is supported by the public interest in our most recent publication on the role of breastfeeding in the neonate immunometabolism and obesity. It has been featured in Science, Science Translational Medicine, and The Journal of Clinical Investigation. To increase clinical utility of our research, we also offer free access to our image analysis software (BeAR©), designed for evaluation of obesity in histopathological specimens. Read more in our Google knowledge panel.
Röszer, T. (2020) The M2 Macrophage. Springer International Publishing. ISBN 978-3-030-50479-3.
https://www.springer.com/gp/book/9783030504793
Yu H, Dilbaz S, Coßmann J, Hoang AC, Diedrich V, Herwig A, Harauma A, Hoshi Y, Moriguchi T, Landgraf K, Körner A, Lucas C, Brodesser S, Balogh L, Thuróczy J, Karemore G, Kuefner MS, Park EA, Rapp C, Travers JB and Röszer T (2019) Breast milk alkylglycerols sustain beige adipocytes through adipose tissue macrophages. J Clin Invest 129(6) DOI 1172/JCI125646
Röszer, T (2018) Understanding the Biology of Self-Renewing Macrophages. Cells 2018, 7(8):103
Waqas SFH, Noble A, Hoang AC, Ampem G, Popp M, Strauß S, Guille M. Röszer T (2017) Adipose tissue macrophages develop from bone marrow-independent progenitors in Xenopus laevis and mouse. J Leukoc Biol 102(3): 845-855
Waqas SFG, Hoang AC, Lin Y, Ampem G, Azegrouz H, Balogh L, Thuróczy J, Chen J, Gerling IC, Nam S, Lim J, Martinez-Ibanez J, Real JT, Paschke S, Quillet R, Ayachi S, Simonin F, Schneider M, Brinkman J, Lamming DW, Seroogy CM, Röszer T (2017) Neuropeptide FF increases M2 activation and self-renewal of adipose tissue macrophages. J Clin Invest 127(7): 2842-2854
Menendez-Guiterrez MP, Röszer T, Fuentes L, Nunez V, Escolano A, Redondo JM, DeClerck N, Metzger D, Valledor AF, Ricote M (2015) Retinoid X receptors orchestrate osteoclast differentiation and postnatal bone remodeling. J Clin Invest 125(2): 809-823
These files allow interactive access to a next generation sequencing dataset of mRNA expression in fat storing (white adipose tissue) and fat oxidizing/thermogenic adipose tissue (brown adipose tissue) in mice. When using this dataset, cite this reference:
Hoang, Anh C., Haidong Yu, and Tamás Röszer. 2021. "Transcriptional Landscaping Identifies a Beige Adipocyte Depot in the Newborn Mouse" Cells 10, no. 9: 2368.
