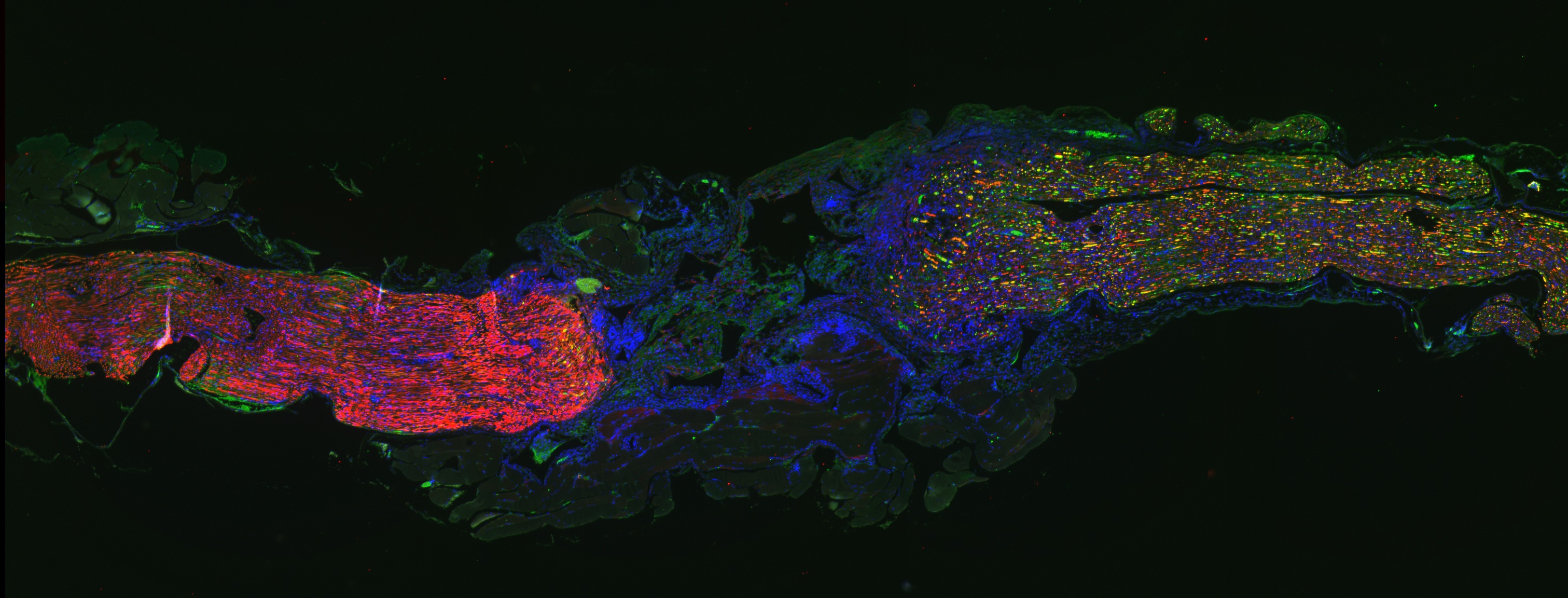
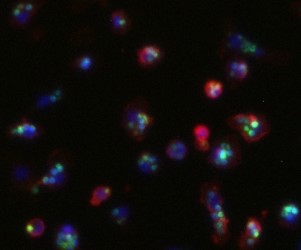
C08: Glucocorticoids influence Schwann cell and macrophage responses in a mouse model of peripheral nerve injury
PIs: S. Vettorazzi, S. Meyer zu Reckendorf
Peripheral nerve injury (PNI) affects 5% of all admitted trauma patients. Regeneration potential and functional recovery of peripheral nerves varies depending on age, injury severity and location. Schwann cells (SCs) are the main drivers of peripheral nerve regeneration after injury by switching from a differentiated to a regenerative repair state. In general, PNI response can be separated into 2 phases: the initial degeneration phase (Wallerian degeneration) and the following regeneration phase. During the degeneration phase, SCs acquire their repair state, which is characterized by morphological changes, myelin breakdown and secretion of growth factors, cytokines and chemokines. As a result, inflammatory cells (i.e. macrophages, granulocytes) are attracted to the injury site to clear tissue debris. Efficient phagocytosis of myelin and axon debris by the attracted macrophages is a crucial step in the degeneration phase to ensure a successful following regeneration phase. In this second phase (regeneration) axons regrow to reinnervate their target tissues and SCs re-differentiate to allow remyelination and therefore full functional recovery. Previous studies could identify glucocorticoids (GCs) as factors that can enhance myelin formation by SCs via stimulating myelination markers (PMP22 and P0). Our investigations show that GCs alone and even more in combination with β-2-adrenergic agonists enhance the phagocytotic capacity of macrophages. Adrenalectomized mice lacking endogenous GC production show an impaired SC myelin formation after PNI, that could be rescued by GC treatment. The efficient and rapid switch of SCs initially to the repair and subsequently to the re-differentiated state is of vital importance for nerve recovery. Just recently, S1P (Sphingosine 1 phosphate) was shown to be a crucial activator of the SC myelinating state. Interestingly, GC treatment can enhance S1P secretion from macrophages in a model of acute lung injury, resulting in a more efficient resolution of inflammation. Furthermore, GCs increase S1P production in macrophages in vitro. Therefore, here we aim to investigate the hypothesis that GCs influence S1P mediated SCs and macrophage responses in vivo in a mouse model of PNI.
Principle investigators
PD Dr. rer. nat. habil. Sabine Vettorazzi
Institut für Molekulare Endokrinologie der Tiere
Zentrum für Biomedizinische Forschung Universität Ulm
Helmholtzstr. 8/1
89081 Ulm
Tel.: +49 731 50 32631
E-Mail: sabine.vettorazzi(at)uni-ulm.de
Dr. rer. nat. Sofia Meyer zu Reckendorf
Institut für Neurobiochemie
Universität Ulm
Albert-Einstein-Allee 11
89081 Ulm
Tel.: +49 731 500 22897
E-Mail: sofia.meyer-zu-reckendorf(at)uni-ulm.de