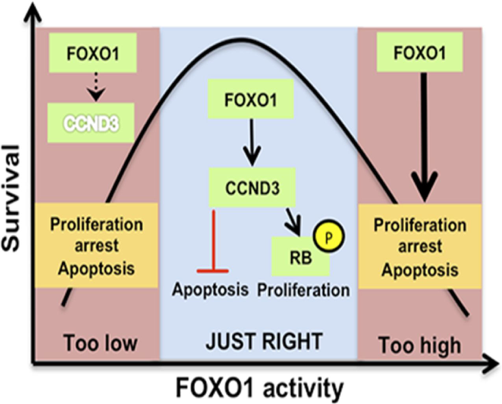
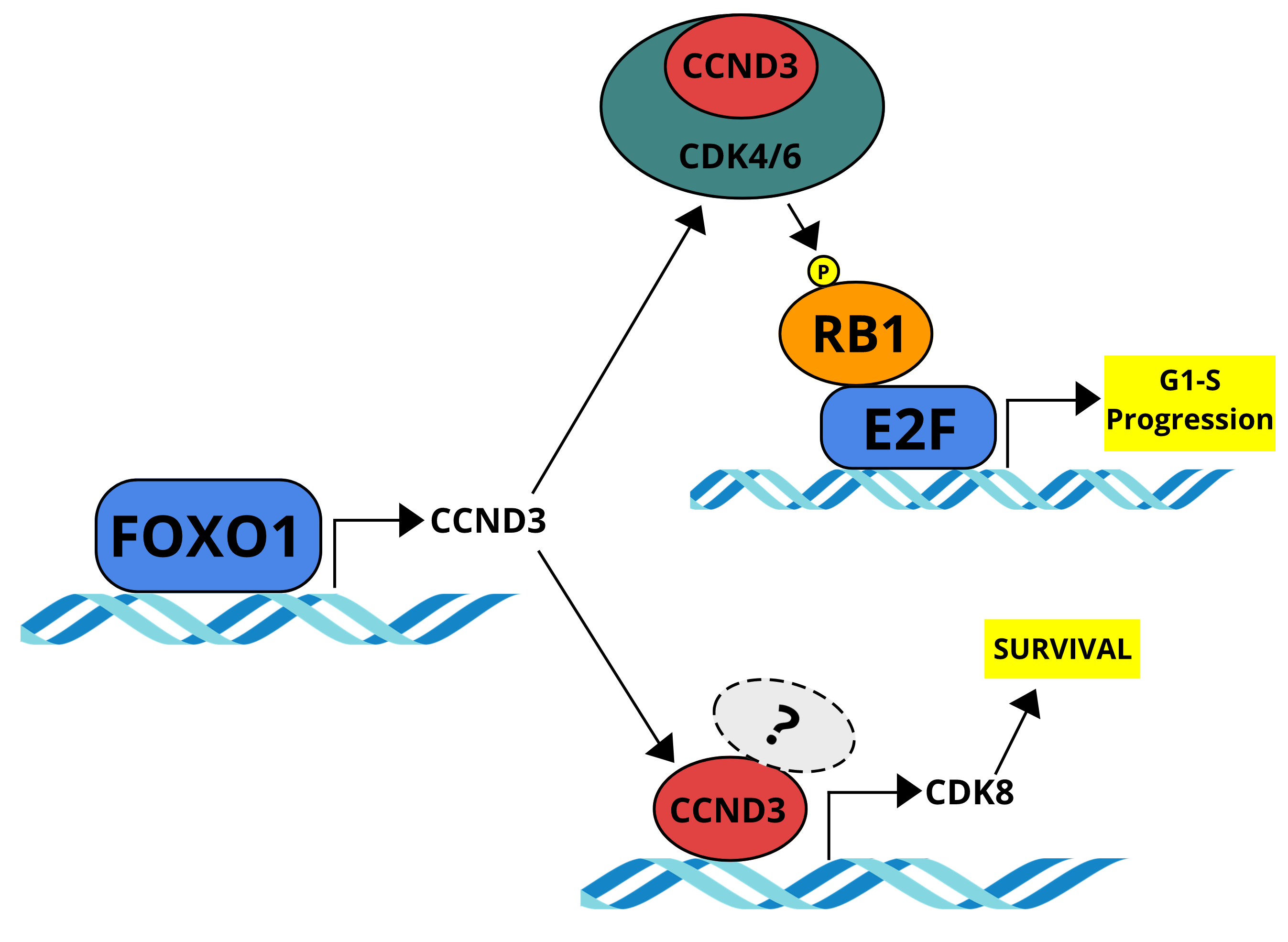
CCND3 is indispensable for the maintenance of B-cell acute lymphoblastic leukemia
Ketzer F, Abdelrasoul H, Vogel M, Marienfeld R, Müschen M, Jumaa H, Wirth T, Ushmorov A.
Oncogenesis. 2022 Jan 10;11(1):1.doi: 10.1038/s41389-021-00377-0.
Hyperstable SGK1 steps out of AKT’s shadow
Gehringer, F., & Ushmorov, A.
Blood.2021 Sep 16;138(11):917-918.doi: 10.1182/blood.2021012518
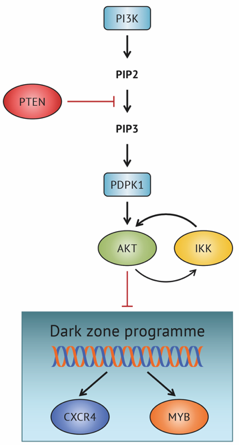
Physiological levels of the PTEN-PI3K-AKT axis activity are required for maintenance of Burkitt lymphoma.
Gehringer F, Weissinger SE, Möller P, Wirth T, Ushmorov A.
Leukemia. 2019 Nov 12. doi: 10.1038/s41375-019-0628-0.
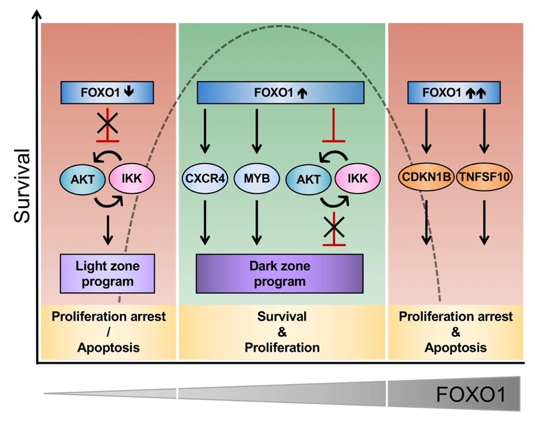
FOXO1 Confers Maintenance of the Dark Zone Proliferation and Survival Program and Can Be Pharmacologically Targeted in Burkitt Lymphoma.
Gehringer F, Weissinger SE, Swier LJ, Möller P, Wirth T, Ushmorov A.
Cancers (Basel). 2019 Sep 25;11(10). pii: E1427. doi: 10.3390/cancers11101427.
FOXO in B-cell lymphopoiesis and B cell neoplasia.
Ushmorov A, Wirth T.
Semin Cancer Biol. 2018 Jun;50:132-141.
Tight regulation of FOXO1 is essential for maintenance of B-cell precursor acute lymphoblastic leukemia.
Wang F, Demir S, Gehringer F, Osswald CD, Seyfried F, Enzenmüller S, Eckhoff SM, Maier T, Holzmann K, Debatin KM, Wirth T, Meyer LH, Ushmorov A.
Blood. 2018 Apr 5. pii: blood-2017-10-813576. doi: 10.1182/blood-2017-10-813576.
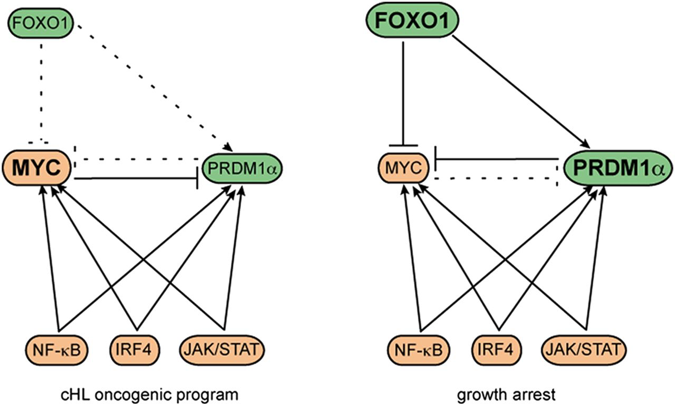
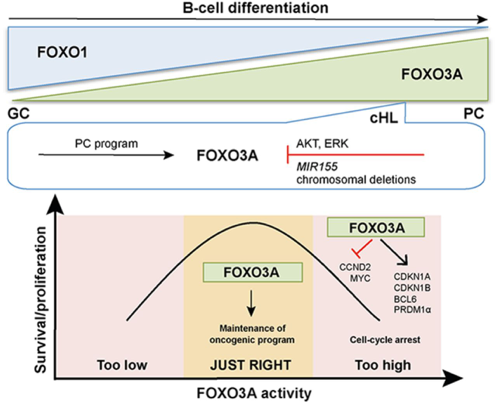
Fine-tuning of FOXO3A in cHL as a survival mechanism and a hallmark of abortive plasma cell differentiation.
Osswald CD, Xie L, Guan H, Herrmann F, Pick SM, Vogel MJ, Gehringer F, Chan FC, Steidl C, Wirth T, Ushmorov A.
Blood. 2018 Apr 5;131(14):1556-1567. doi: 10.1182/blood-2017-07-795278.
Activation of oncogenic pathways in classical Hodgkin lymphoma by decitabine: A rationale for combination with small molecular weight inhibitors.
Swerev TM, Wirth T, Ushmorov A.
Int J Oncol. 2017 Feb;50(2)
FOXO in B-cell lymphopoiesis and B cell neoplasia.
Ushmorov A, Wirth T.
Semin Cancer Biol. 2017 Jul 31.