Dissociative Adsorption of Hydrogen on Pd(100)
Contents
Introduction
Hydrogen Dissociation on the Clean Pd surface
Illustration of the Steering Effect
Poisoning of the Hydrogen Dissociation by a Sulfur Adlayer
Publications
Introduction
Understanding reactions on surfaces plays an important role in a wide range technologically relevant applications. Among those are the heterogenous catalysis - the majority of reactions in the chemical industry employ catalysts; crystal growth, which determines, e.g., the quality of semiconductor devices; corrosion and lubrication , which influences the durability of mechanical systems; or hydrogen storage in metals, just to mention a few. The reactions involved in these processes are often too complicated to be studied in detail as a whole. Therefore in surface science one tries to understand reaction mechanisms by breaking up complicated reactions into simpler steps which are then studied under well-defined conditions.
Reactions on well-defined surfaces still occur on a multi-dimensional interaction potential. Up to recently the reaction dynamics had been described in low-dimensional studies on model potentials. This was due to the lack of reliable potential energy surfaces and the large computational effort for high-dimensional dynamics studies. This situation has changed significantly within the last five years caused by the increase in computer power and the development of efficient algorithms. For the paradigm of simple reactions on surfaces - the dissociation of hydrogen on metal surfaces - detailed potential energy surfaces obtained by density functional theory calculations are now available, and dynamical studies treating explicitly all hydrogen degrees of freedom have been performed.
Hydrogen Dissociation on the Clean Pd surface
We have studied the dissociative adsorption of hydrogen on Pd(100) by ab initio quantum dynamics and ab initiomolecular dynamics calculations. Treating all hydrogen degrees of freedom as dynamical coordinates implies a high dimensionality and requires statistical averages over thousands of trajectories. An efficient and accurate treatment of such extensive statistics is achieved in a three-step approach: In a first step we evaluate the ab initio potential energy surface (PES) for a number of appropriate points in configuration space. Then (as step 2) we determine an analytical representation which serves as an interpolation between the actually calculated points. In an independent third step dynamical calculations are performed on the analytical representation of the PES. Thus the dissociation dynamics is investigated without any crucial assumption except for the Born-Oppenheimer approximation which is anyhow employed when density-functional theory calculations are performed.
Figure 1 presents six-dimensional quantum dynamical calculations of the sticking probability as a function of the kinetic energy of a H2 beam under normal incidence on a Pd(100) surface and five-dimensional calculations for D2. In addition, the results of the H2 molecular beam experiment by Rendulic, Anger and Winkler, Surf. Sci. 208, 404 (1989), are shown.
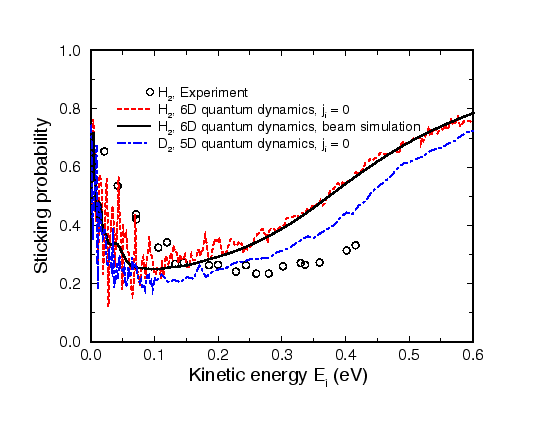
Quantum effects are non-neglible for hydrogen dissociation on surfaces. The discrete nature of diffraction and molecular excitation leads to a strong oscillatory structure of the sticking probability as a function of the incident kinetic energy. Furthermore, zero-point effects cause substantial deviations between the averaged quantum dynamical calculations and the classical calculations.
The potential energy surface (PES) of the interaction of hydrogen with Pd(100) has non-activated paths towards dissociative adsorption and no molecular adsorption well. However, the majority of pathways towards dissociative adsorption has in fact energy barriers with a rather broad distribution of heights and positions, i.e. the PES is strongly anisotropic and corrugated. A slow molecule moving on such a PES with an unfavorable initial configuration for dissociative adsorption can be steered efficiently towards non-activated paths to adsorption by the forces acting upon the molecule. This mechanism becomes less efficient at higher kinetic energies because then the molecule is too fast to be diverted significantly. More particles are therefore scattered back into the gas-phase from the repulsive part of the potential. This leads to the initial decrease of the sticking probability. At even higher kinetic energies the molecule will eventually have enough energy to directly traverse the barriers causing the sticking probability to rise again. The steering effect is illustrated by classical trajectories in the next section.
Illustration of the Steering Effect
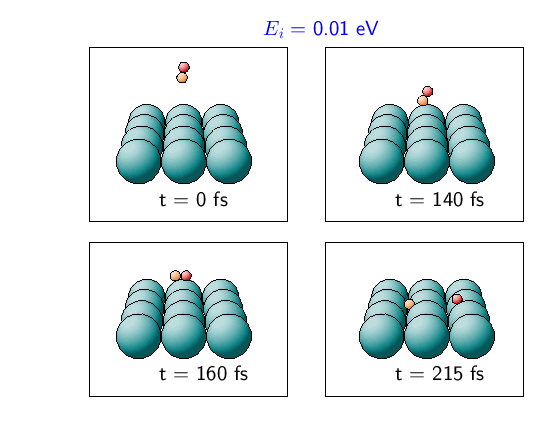
In order to illustrate the steering effects, we use the results of two typical classical trajectory runs. This is done in the next two figures, where snapshots of these two trajectories are shown. Figure 2 illustrates the steering effect. The incident kinetic energy is Ei = 0.01 eV. Initially the molecular axis is almost perpendicular to the surface. In such a configuration the molecule cannot dissociate at the surface. But the molecule is so slow that the forces acting upon it can reorient the molecule. It is turned parallel to the surface and then follows a non-activated path towards dissociative adsorption.
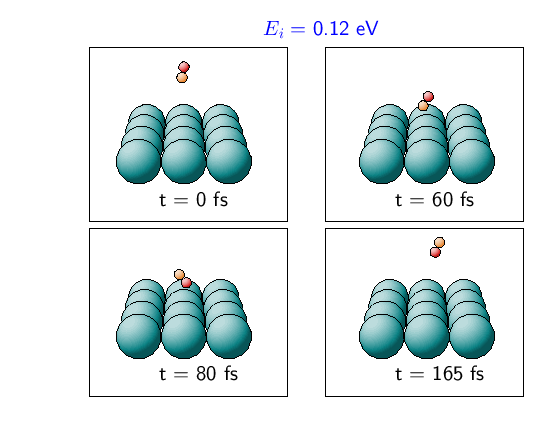
In the case of Figure 3, the initial conditions are the same as in Figure 2, except that the molecule has a higher kinetic energy of 0.12 eV. Due to the anisotropy of the PES the molecule also starts to rotate to a configuration parallel to the surface. However, now the molecule is so fast that it hits the repulsive wall of the potential before it is in a favorable configuration to dissociative adsorption. At the classical turning point there is a very rapid rotation corresponding to a flip-flop motion, and then the molecule is scattered back into the gas-phase rotationally excited.
Poisoning of the Hydrogen Dissociation by a Sulfur Adlayer
The presence of an adsorbate on a surface can profoundly change the surface reactivity. An understanding of the underlying mechanisms and their consequences on the reaction rates is of decisive importance for, e.g., designing better catalysts. Typically the breaking of molecular bonds is the rate-limiting step in a catalytic reaction. On Pd(100) sulfur preadsorption leads to the poisoning of hydrogen dissociation, i.e., the hydrogen dissociation is no longer non-activated as on the clean Pd(100) surface. Density functional theory calculations have shown that hydrogen dissociation on sulfur-covered Pd(100) is still exothermic, however, the dissociation is hindered by the formation of energy barriers in the entrance channel of the potential energy surface (PES).
Figure 4 compares our results for the sticking probability as a function of the kinetic energy of the incident hydrogen beam with the experiment (Rendulic, Anger and Winkler, Surf. Sci. 208, 404 (1989)). The calculated sticking probabilities are significantly larger than the experimental results. We believe that they are mainly due to uncertainties in the experimental characterization of the sulfur coverage. The onset of dissociative adsorption at Ei = 0.1 eV, however, is well reproduced by the calculations. The potential energy surface shows an huge corrugation and anisotropy. This causes significant zero-point effects in the quantum dynamics. Since these zero-point effects are absent in classical dynamics, the classical results are larger than the quantum ones.
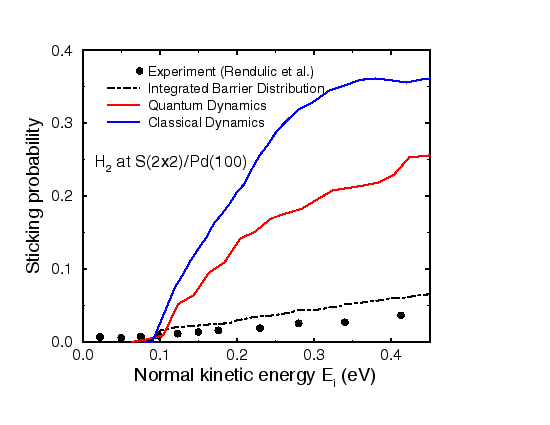
In Figure 4 also the integrated barrier distribution is plotted which is the fraction of the configuration space for which the barrier towards dissociation is less than E; it corresponds to the sticking probability in the classical sudden approximation which forms the basic approximation behind the so-called hole model. A comparison of the dynamical results with the integrated barrier distribution shows that the sticking probabilities are much larger than what one would expect from the hole model. We have carefully investigated the dissociation dynamics by analysing swarms of trajectories with different initial conditions. Some of them are shown in Figure 5.
The trajectories correspond to molecules that are incident along the diagonal of the surface unit cell of the S(2x2)/Pd(100) surface with a kinetic energy of Ei = 0.3 eV. In the hole model none of the incident conditions would lead to a dissociation event. The sulfur atoms are strongly repelling the hydrogen molecules already rather far away from the surface. The molecules directly hitting the sulfur atoms are scattered back into the gas-phase, but the other molecules are focused into the center of the surface unit cell. This diversion of the molecular center-of-mass is accompanied by a reorientation of the molecular axis along the [011] troughs of the surface. Thus steering causes the surprising result that actually at this energy the sticking probability at the (2x2) sulfur-covered Pd(100) surface is almost as large as at the clean Pd(100) surface in spite of the fact that at the clean surface the fraction of open dissociation pathways is more than five times larger for this energy.
Publications
- S. Wilke, D. Hennig, and R. Loeber, Ab initio calculations of hydrogen adsorption on (100) surfaces of palladium and rhodium, Phys. Rev. B 50, 2548-2560 (1994).
- S. Wilke, D. Hennig, R. Löber, M. Methfessel, and M. Scheffler, Ab-initio study of hydrogen adsorption on Pd(100), Surf. Sci. 307-309, 76-81 (1994).
- A. Groß, The role of lateral surface corrugation for the quantum dynamics of dissociative adsorption and associative desorption, J. Chem. Phys. 102, 5045-5058 (1995).
- A. Groß, S. Wilke, and M. Scheffler, Six-dimensional quantum dynamics of adsorption and desorption of H2 at Pd(100): Steering and steric effects, Phys. Rev. Lett. 75, 2718-2721 (1995).
- S. Wilke and M. Scheffler, Poisoning of Pd(100) for the dissociation of H2: a theoretical study of co-adsorption of hydrogen and sulphur, Surf. Sci. 329, L605-L610 (1995).
- A. Gross and M. Scheffler, Influence of molecular vibrations on dissociative adsorption, Chem. Phys. Lett. 256, 417-423 (1996).
- A. Gross and M. Scheffler, Scattering of hydrogen molecules from a reactive surface: strong off-specular and rotationally inelastic diffraction. Chem. Phys. Lett. 263, 567-573 (1996).
- A. Gross and M. Scheffler, Steering and ro-vibrational effects in the dissociative adsorption and associative desorption of H2/Pd(100), Prog. Surf. Sci. 53, 187-196 (1996).
- A. Gross, S. Wilke, and M. Scheffler, Six-dimensional quantum dynamics of adsorption and desorption of H2 at Pd(100): no need for a molecular precursor adsorption state, Surf. Sci. 357-358, 614-618 (1996).
- S. Wilke and M. Scheffler, Mechanism of poisoning the catalytic activity of Pd(100) by a sulfur adlayer, Phys. Rev. Lett. 76, 3380-3383 (1996).
- S. Wilke and M. Scheffler, Potential energy surface for H2 dissociation over Pd(100), Phys. Rev. B 53, 4926-4932 (1996).
- A. Gross and M. Scheffler, Role of zero-point effects in catalytic reactions involving hydrogen. J. Vac. Sci. Technol. A 15, 1624-1629 (1997).
- A. Gross and M. Scheffler, Ab initio quantum and molecular dynamics of the dissociative adsorption of hydrogen on Pd(100), Phys. Rev. B 57, 2493 (1998).
- C.M. Wei, A. Gross, and M. Scheffler, Ab initio calculation of the potential energy surface for the dissociation of H2 on the sulfur covered Pd(100) surface. Phys. Rev. B 57, 15572 (1998).
- A. Gross, C.M. Wei, and M. Scheffler, Poisoning of Hydrogen Dissociation at Pd (100) by Adsorbed Sulfur Studied by ab initio Quantum Dynamics and ab initio Molecular Dynamics, Surf. Sci. 416, L1095-L1100 (1998).
- A. Groß, Hydrogen dissociation on metal surfaces -- a model system for reactions on surfaces, Appl. Phys. A 67, 627-635 (1998).
[Preprint, Springer Online]
This page was created by Axel Groß.
