Pfister lab - research
The nucleolar stress response in health and disease
Our research is dedicated to unraveling molecular mechanisms of cellular signaling pathways connected to growth and survival. We have a particular focus on the nucleolar stress response and its profound connection to apoptosis and autophagy, respectively.
Functional ribosomes are key to proper cell growth and survival. They are formed in a process termed ribosome biogenesis, which takes place in the sub-nuclear structure, the nucleoli. Defective ribosome biogenesis leads to nucleolar stress - a situation, which is linked to multiple downstream consequences that range from DNA damage, to cell cycle arrest, apoptosis and autophagy (Pfister, 2021 & Pfister, 2019). Moreover, the Wnt signaling pathway drives ribosome biogenesis and is involved in a feedback mechanism that couples activation of the Wnt signaling pathway in response to nucleolar stress (Dannheisig et al., 2021).
With its cellular consequences, the nucleolar stress response functions as intriguing double-edged sword: In pathological situations, nucleolar stress can increase cancer incidence and is linked to neurodegeneration and aging. Mutations in ribosomal proteins or ribosome biogenesis factors are coupled to ribosomopathy syndromes with a variety of symptoms such as craniofacial cartilage defects and, again, increased cancer incidence.
However, in chemotherapy, induction of nucleolar stress is beneficial to eliminate tumor cells. This renders the nucleolar stress response as an attractive candidate for investigating diverse therapeutical interventions.
Thus, we investigate the role of specific ribosome biogenesis factors and small molecule compounds in the nucleolar stress response. To do so, we screen for the function of chemical compounds in the nucleolar stress response.
To gain novel insights into the multifaceted nucleolar stress response, we follow a multidisciplinary approach. We integrate cell and molecular biology, biochemistry and developmental biology techniques by using cell culture and the classical model organism Xenopus laevis. Collaborative screening methodologies are another part of our studies, to elucidate intricate signaling networks. Biomedical questions and applications are studied in more detail together with our collaborators.
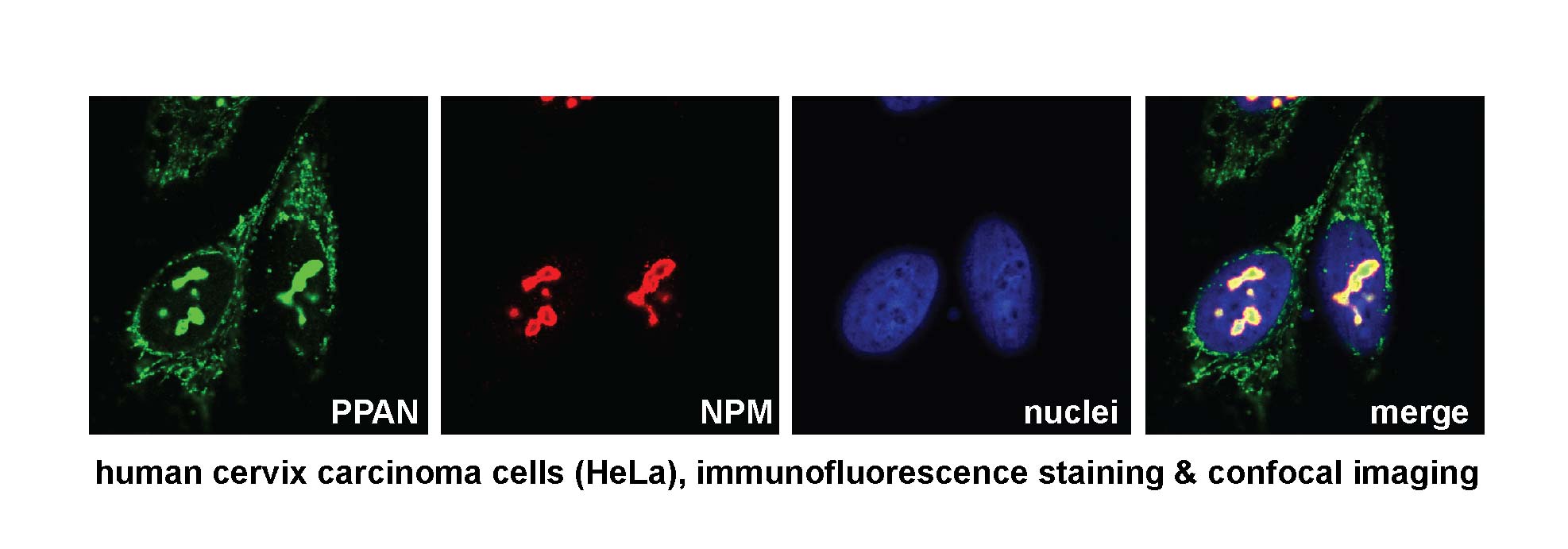
Our previous research on the ribosome biogenesis factor PPAN (Peter Pan).
We previously demonstrated that the nucleolar protein PPAN (Peter Pan) interacts with NPM (Nucleophosmin), which is commonly mutated in AML (Pfister et al., 2015). Both factors are Wnt target genes and are crucial for cell growth and survival, as they regulate ribosome biogenesis in the nucleolus.
PPAN knockdown mediates cell cycle defects, triggers apoptosis and apoptosis-related DNA damage independently of p53 (Pfister et al., 2015; Keil et al., 2019). In contrast, PPAN overexpression made cancer cells more resistant to treatment with chemotherapeutic agents. We could further demonstrate that PPAN protein translocates from the nucleus into mitochondria and can interact with the mitochondrial membrane lipid, cardiolipin. (Dannheisig et al., 2019).
We found that PPAN is involved in the homeostasis of mitochondrial architecture. We further demonstrated that loss of PPAN leads to severe defects in mitochondrial functionality and impairs mitochondrial inner membrane structure, induces MOMP (mitochondrial outer membrane permeabilization), all of which results in efflux of Cytochrome C and triggers apoptosis (Pfister et al., 2015; Keil et al., 2019; Dannheisig et al., 2019). Moreover, knockdown of PPAN increases autophagic flux and induces mitophagy in a Parkin-dependent manner (Dannheisig et al., 2019).
Nucleolar stress activates downstream signaling pathways such as autophagy and Wnt/ beta-Catenin signaling
We have previously shown that induction of nucleolar stress activates autophagic flux and stimulates expression of core autophagy regulators already at mRNA level (Dannheisig et al., 2021a and Pfister, 2023).
Wnt/b-catenin signaling drives the expression of target genes regulating e.g. proliferation, migration and apoptosis. De-regulation of Wnt signaling has severe consequences leading to tumors, for instance colon cancer and leukaemia. In contrast, increasing evidence suggests that Wnt/b-catenin signaling is down-regulated during aging, e.g. in hematopoietic stem cells.
We previously uncovered that a class of nucleolar ribosome biogenesis factors are involved in the regulation of Wnt signaling. Interestingly, induction of nucleolar stress by chemical compounds or knockdown and CRISPR/Cas9 strategies activates Wnt signaling (Dannheisig et al., 2021a).
Funded by:
Medical Faculity Ulm University, Bausteinprogramm
Deutsche Krebshilfe
