Imagine a big city without a car driving and no mail being delivered. The resulting chaos is similar to what happens to a cell when its molecular transport systems are impaired. Our goal is to understand the molecular principles underlying cargo recognition by transport complexes, complex assembly and activation, and eventually complex disassembly after the transport. Our research tools are X-ray crystallography, quantitative biophysical approaches, biochemistry, and in vivo studies.
RNA localization in yeast: One model we work with is Saccharomyces cerevisiae, in which we analyze the directional transport of ASH1 mRNA during mitosis. Besides mRNA, this cargo-transport complex consists of the myosin motor Myo4p, its bound adapter She3p, and the RNA-cargo binding protein She2p. We determined several crystal structures of these core factors alone and in complex. These experiments are complemented by functional analysis of reconstituted complexes.
See for instance: Heym et al. J Cell Biol 2013; Niedner et al PNAS 2013 and Edelmann et al. Nat Struct Mol Biol 2017. For an overview article, please visit Niessing et al. WIRE 2018.
RNA localization in higher eukaryotes: Here mRNA-transport particles consist of several dozens of factors, making the in vitro assembly of functional particles much more difficult. For this reason, we concentrate our work on neuronal RNA-binding proteins such as Staufen and Pur-alpha.
See for instance Graebsch et al. PNAS 2009; Heber et al. Nature Commun. 2019.
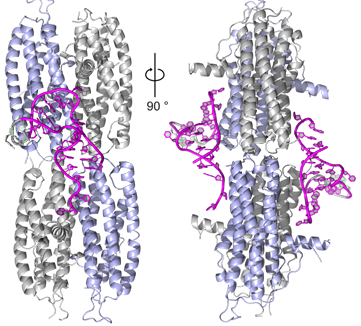
The PURA Syndrome: Mutations in Pur-alpha have recently been shown to be the cause of the sporadic monogenic PURA Syndrome. With crystal structures at hand, we can provide interpretations of the molecular effects such mutations have on protein folding and function. Together with our partner lab at the Helmholtz Zentrum München, we aim to understand the interaction networks Pur-alpha is involved in and the effects of disease-causing mutations on these networks. In addition, we aim to assess how the two paralogs of PURA, i.e., PURB and PURG influence PURA and what their physiological functions are.
You can find an overview article on this topic here: Molitor et al. 2021. See also Weber et al. Elife 2016, Reijnders et al J Med Genet 2018, and Molitor et al. Nucl. Acids Res. 2023.
Translational control and auto-immunity: Roquin is an RNA-binding protein required for downregulating the expression of co-stimmulatory receptors in T cells and thereby preventing hyperactivation of the immune response. Together with our partner labs of Vigo Heissmeyer (LMU) and Michael Sattler (Helmholtz Zentrum München), we aim to understand how Roquin binds RNA to fulfill its function.
See also Schlundt et al. Nat Struct Mol Biol 2014; Janowski et al Nat Comm 2016; Rehage et al. Nat Comm 2018; Behrens et al. Nature Immunol 2021,
