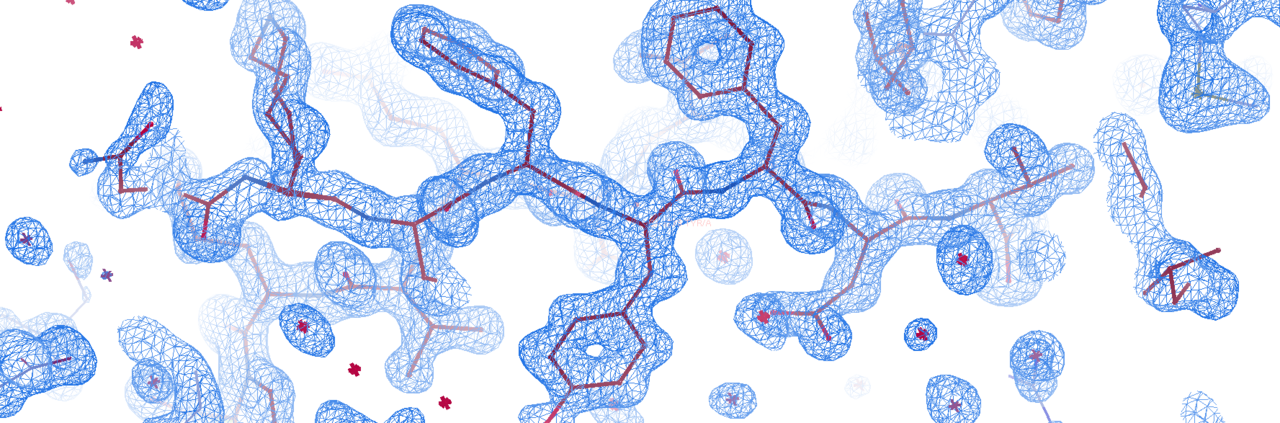
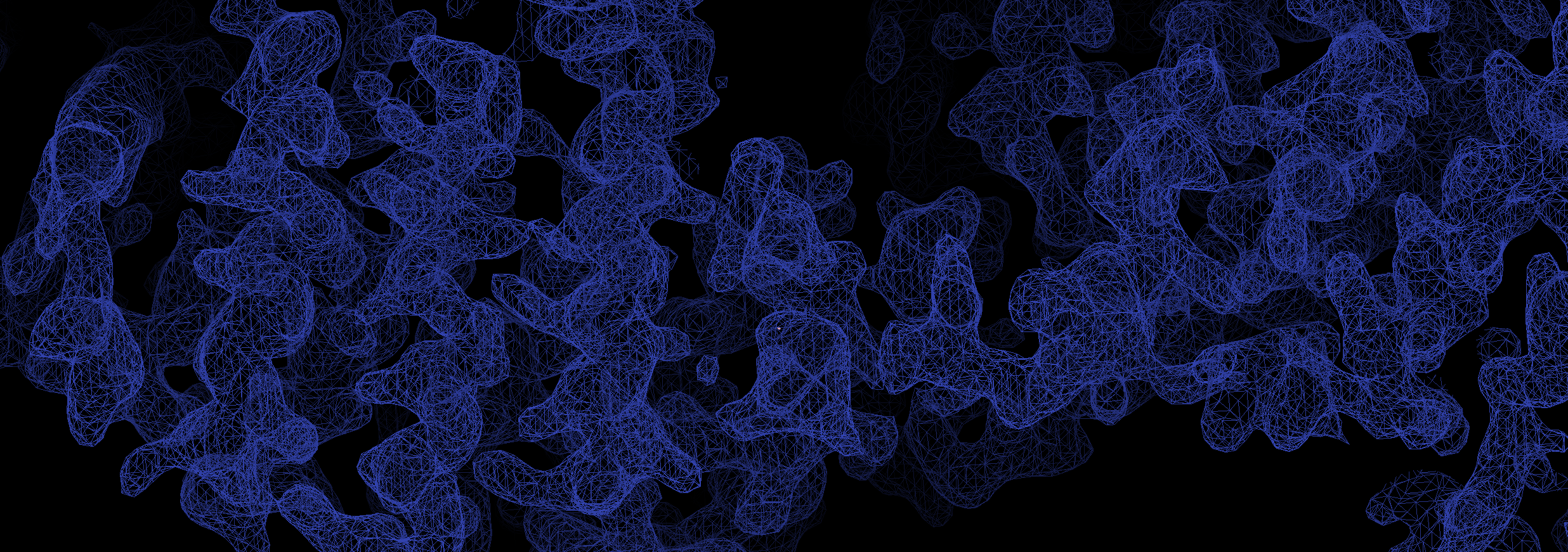
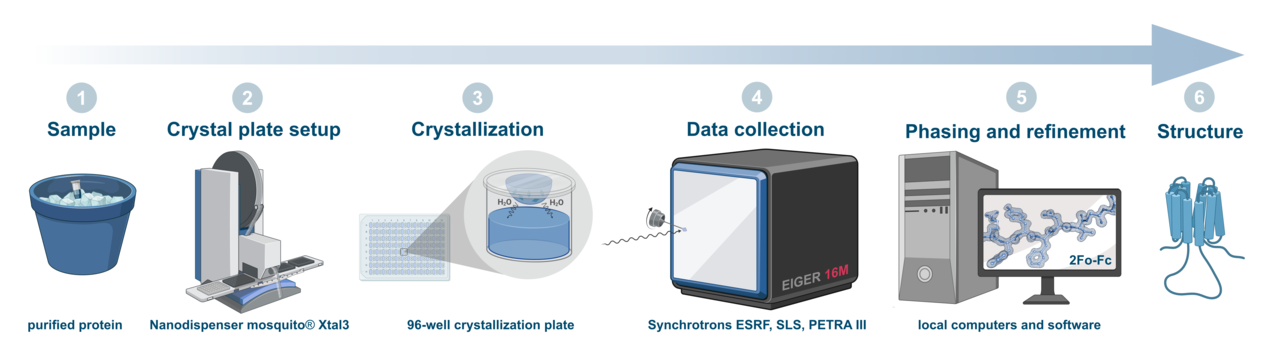
X-ray crystallography is the method of choice for the determination of high-resolution macromolecular structures including protein, DNA, RNA and complexes therof. It is complemented by other techniques such as nuclear magnetic resonance (NMR) as well as cryo-electron microscopy (Cryo-EM). While NMR and Cryo-EM share limitations concerning the size of the molecules investigated, X-ray crystallography delivers structural information irrespective of size and on an atomic level.
With the Institute of Pharmaceutical Biotechnology and the X-ray crystallography facility we are closing a gap in structural biology research at Ulm University. We offer collaborational- or service-like operation modes.
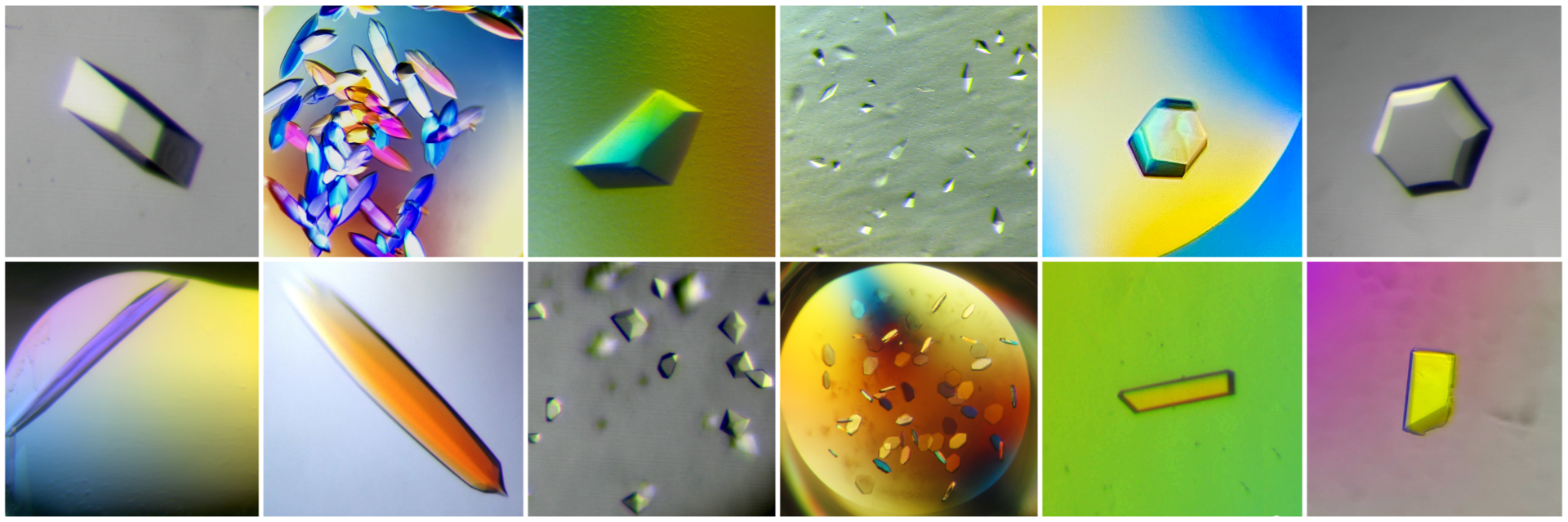
The facility is equipped with a state-of-the-art nanodispenser (Mosquito Xtal3) and a liquid handling station for automated preparation of crystallization conditions and reformating screens (Hamilton MICROLAB Star) and microscopes for the inspection and documentation of crystals. The equipment is complemented by expert knowledge of crystal handling, data collection as well as structure solution.
For X-ray diffraction experiments we have regular access to beamlines of different european synchrotrons like ESRF (Grenoble, France), SLS (Villigen, Switzerland) or DESY (Hamburg, Germany).
The facility is established and run by the Institute of Pharmaceutical Biotechnology (Niessing lab Ulm) and headed by Dr. Thomas Monecke. Please contact us for collaborations, services or more details.
The usage regulations (Nutzungsordnung) of the X-ray crystallography facility can be downloaded here. The facility is listed in the DFG Research Infrastructure portal (DFG RIsources).