Kühl lab - research
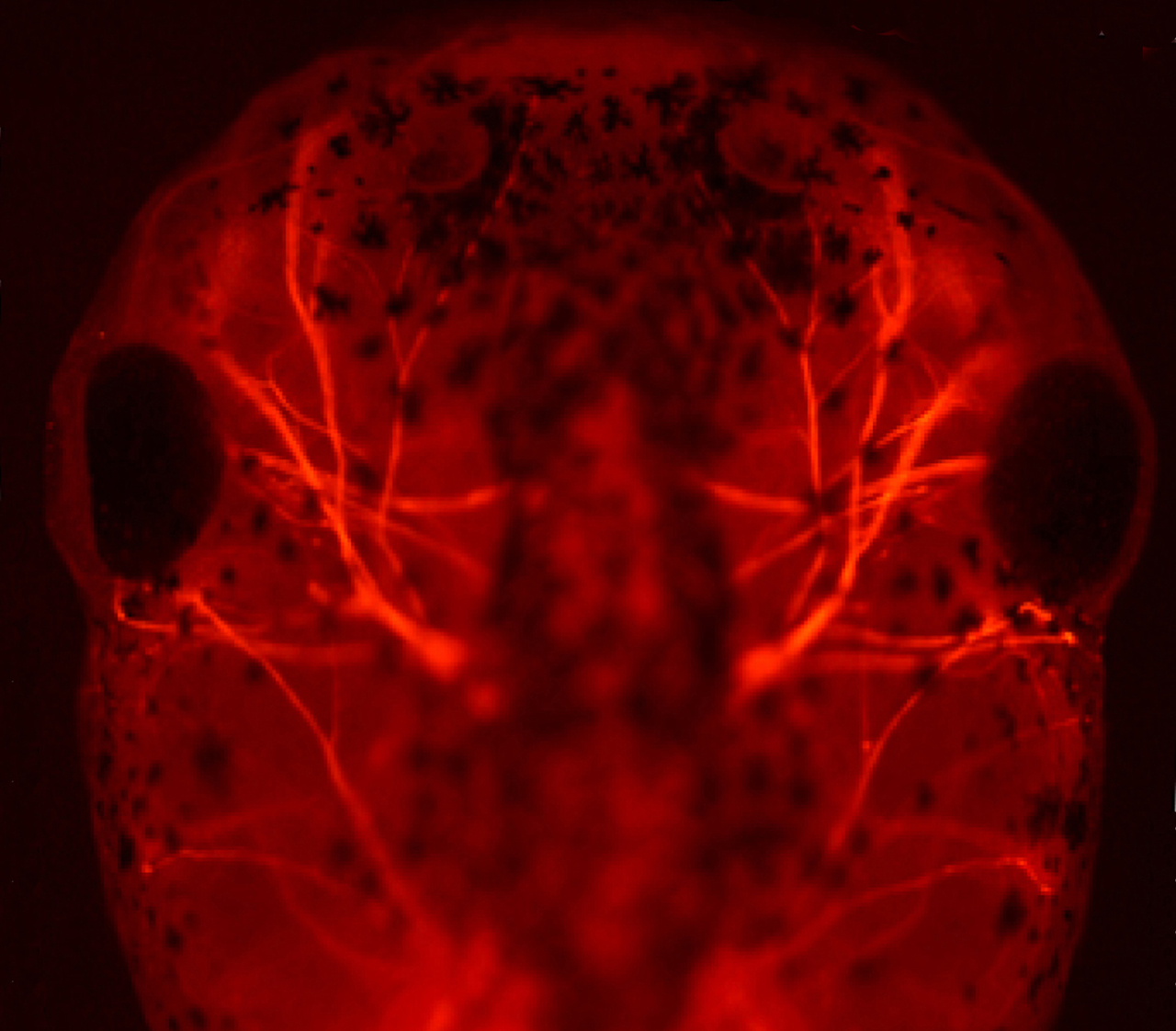
Neural development (Kühl SJ)
During neural development, non-canonical Wnt signalling pathways regulate the expression of selected targeted genes (Maurus et al., EMBO J. 2005). Target genes of non-canonical Wnt are among others Pescadillo, Peter Pan and Alcam (Gessert et al., Dev. Biol. 2007; Bugner et al. Development 2011; Cizelsky et al., Development 2013). We could furthermore show that proteins of the synapse are required for anterior neural development and that some of these molecules balance canonical and non-canonical Wnt pathway (e.g. Rothe et al. Development 2017). In this context, we are currently investigating the molecular mechanisms underlying non-canonical Wnt during neural development in the vertebrate organism Xenopus laevis.
Moreover, we are interested in the mechanisms underlying neurodevelopmental disorders in humans. In collaboration with clinical reseachers, we therefore examine the function of potential candidate genes involved in neurodevelopmental processes during early neural development in the Xenopus leavis embryo (Hempel et al., J. Med. Genet. 2016; Zawerton et al., Am. J. Hum. Genet. 2018). Currently, we investigate candidate genes involved in the Galloway-Mowat-Syndrome (GAMOS) (Mann et al., J. Am. Soc. Nephrol. 2021). A further research field is the analysis of ribosomal proteins during early embryogenesis of Xenopus laevis. Mutations in ribosomal factors can lead to congenital diseases in humans called ribosomopathies. Clinical manifestations include e.g. craniofacial malformations or eye defects. By loss of function approaches, we could already show that inferference with ribosomal factors results in defects during Xenopus embryogenesis and that these factors have diverse roles during development (Gessert et al., Dev. Biol. 2007; Bugner et al. Development; Tecza et al. Biol. Cell, 2011). To get more light in these complex picture, we currently examine further ribosomal factors during Xenopus anterior neural development.
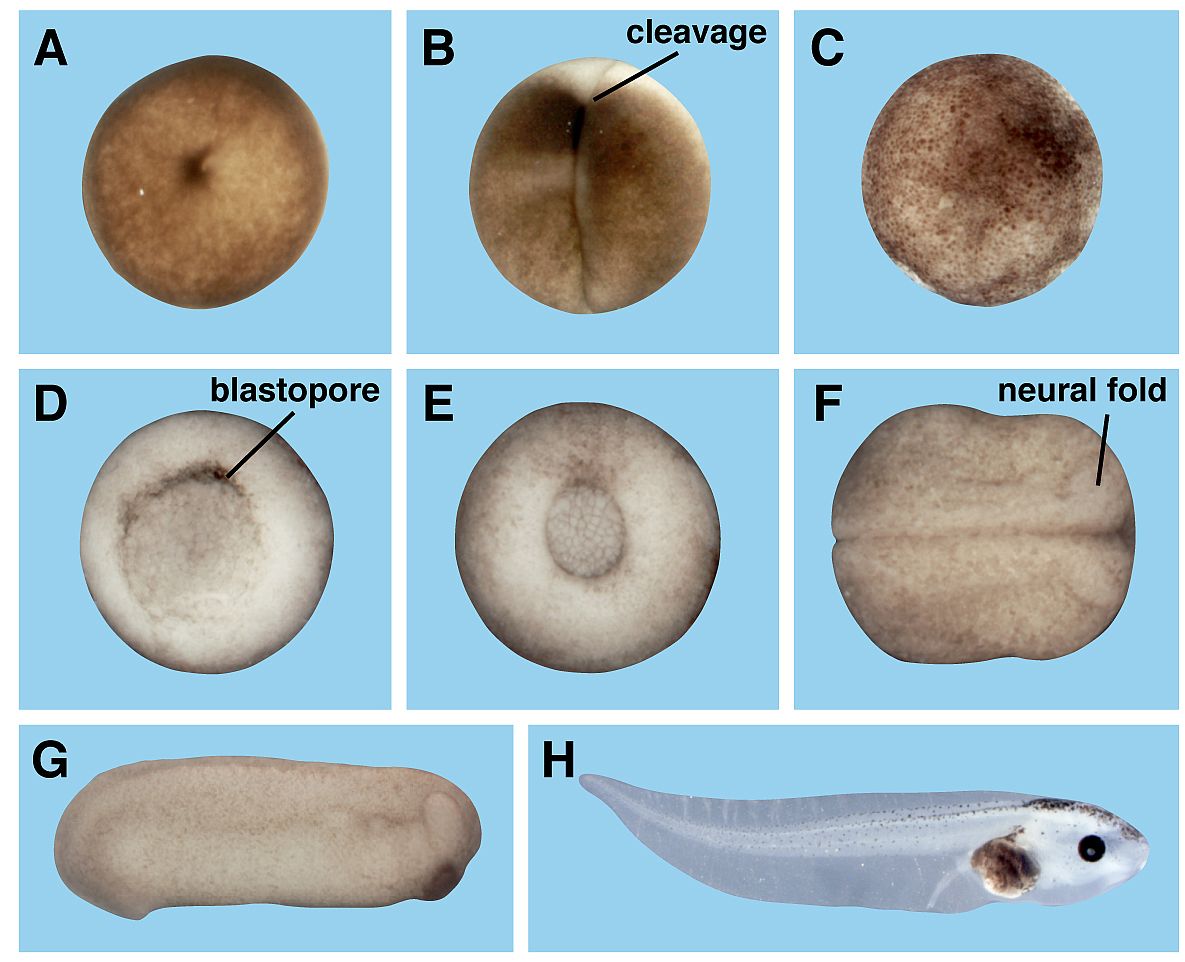
Toxicology and developmental biology (Kühl SJ)
Pesticides, herbicides and insecticides in our environment negatively affect animals such as insects or aquatic animals. In this project, we analyze the influence of different pesticides on the early embryonic development of the aquatic vertebrate model system, the south African claw frog Xenopus laevis.
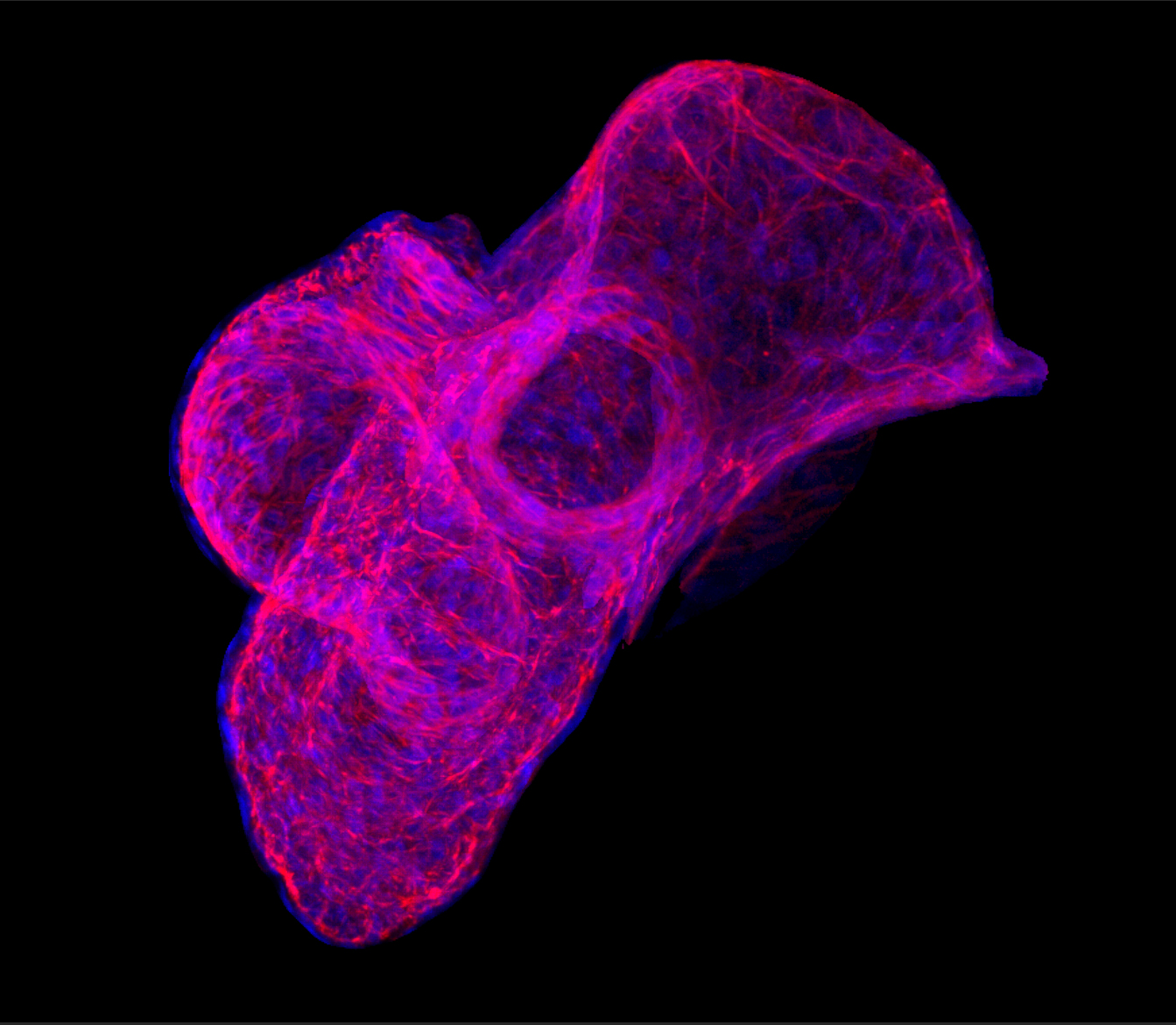
Heart development (Kühl M)
The heart is the first functional organ during mammalian development. Defects in the development of cardiac tissue result in congenital heart diseases, which occur in approximately 1% of all newborns and are estimated to be the cause of 10% of still-births and spontaneous abortions. Defects in regulatory molecules that act early in heart development have been linked to congenital cardiovascular malformation. A detailed analysis of normal heart development at the molecular level will deepen our understanding of pathological changes in congenital heart diseases. Furthermore, the recent identification of adult cardiac stem cells that can differentiate into functional cardiomyocytes opens a new perspective in the therapy of heart diseases in the long run and reinforces the necessity to understand the process of normal cardiac development. We recently have shown that certain Wnt signalling activities are required for vertebrate cardiac development (Pandur et al., Nature 2002; Gessert at al. Dev. Biol. 2008; Gessert and Kühl, Dev. Biol. 2010; Dorn et al. Stem Cells 2015 ). Currently our studies focus on the characterization of gene regulatory networks and extra cellular growth factors during cardiac development.
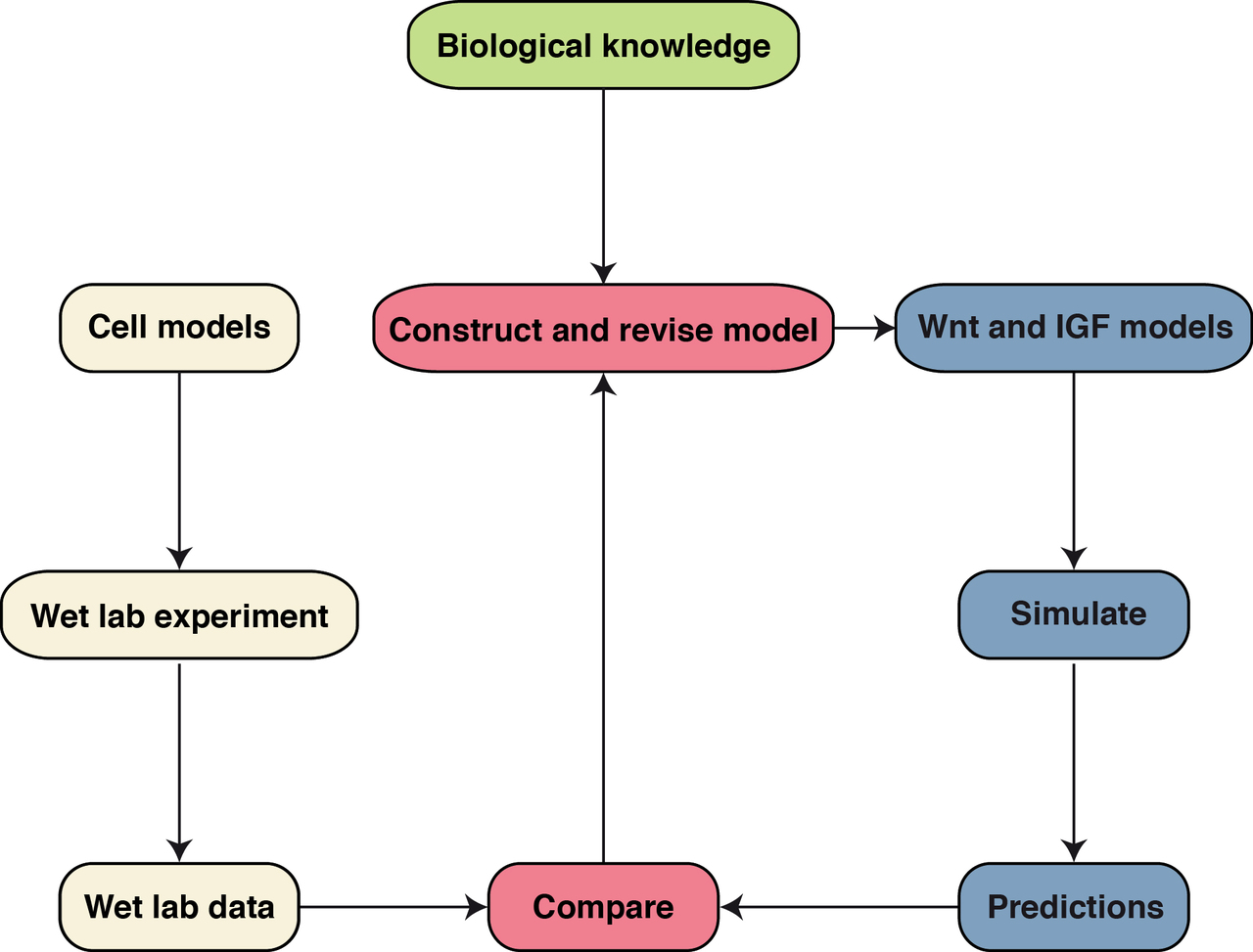
Systems Biology (Kühl M)
Members of the Wnt family of extracellular ligands can activate different intracellular signaling branches that are highly connected and interdependent to form a complex signaling network (Kestler and Kühl, 2008). Several target genes of Wnt signaling regulate Wnt signaling in a negative feedback loop. Taken together this allows for a switch-like or an oscillatory behavior of pathway activity under certain conditions (Wawra et al., 2007; Kestler et al. 2011). This complexity hampers an intuitive understanding of the global behavior of this network in a given cellular context. Mathematical models contribute to our understanding of complex signal transduction circuits and gene regulatory networks and furthermore help to identify causal relationships for changes in signaling in diseases and during aging (Kestler et al. 2008). This a collaboration project with the Institute of Medical Systems Biology (Head: Prof. Dr. Hans Kestler).
