Philipp lab - research
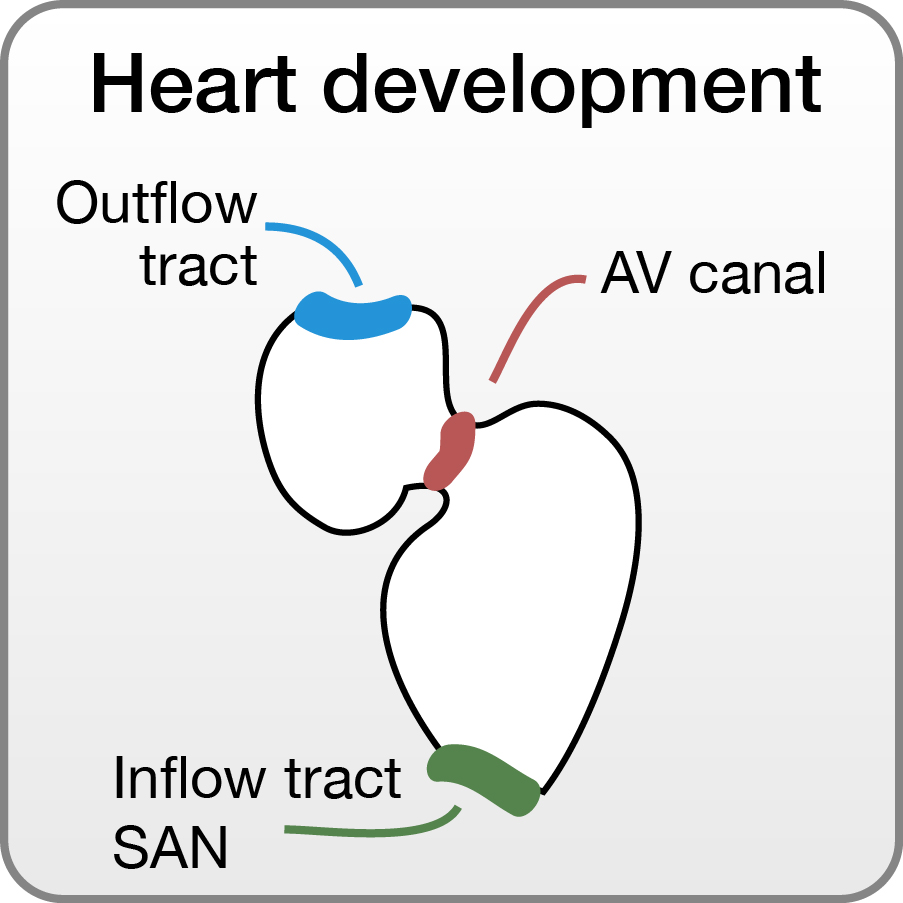
Molecular mechanisms underlying heart development
The heart is the first organ to develop and become functional. It is furthermore the organ that is most prone to developmental malformations which is reflected by the large number of newborns diagnosed with a congenital heart defect. We apply small chemical compound screens, mass spectrometry as well as traditional loss-of-function strategies to contribute to the current knowledge of the molecular mechanisms underlying heart development. As a model we use zebrafish embryos, which develop a simple, yet functional, compartmentalized heart within 48 hours post fertilization.
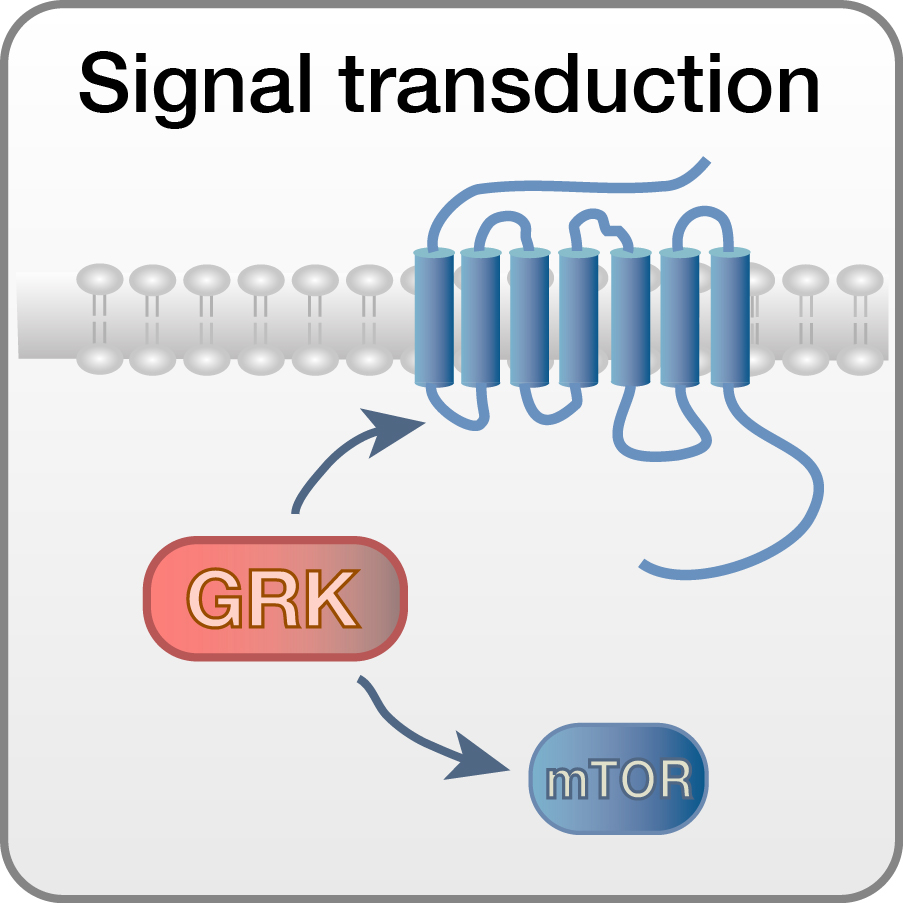
Signaling networks during development
Signal transduction serves as a form of communication between and within cells. We try to explore and better understand the physiological role of signal transduction during embryonic development. How are external signals transduced into a given cell and then between different compartments within a cell? How would a change in such signaling reflect a physiological or pathological state? And how could we manipulate signaling and with that potentially develop new therapies? To address these questions we focus on G protein-coupled receptor kinases (GRKs), which control a multitude of receptor-dependent and –independent signaling pathways.
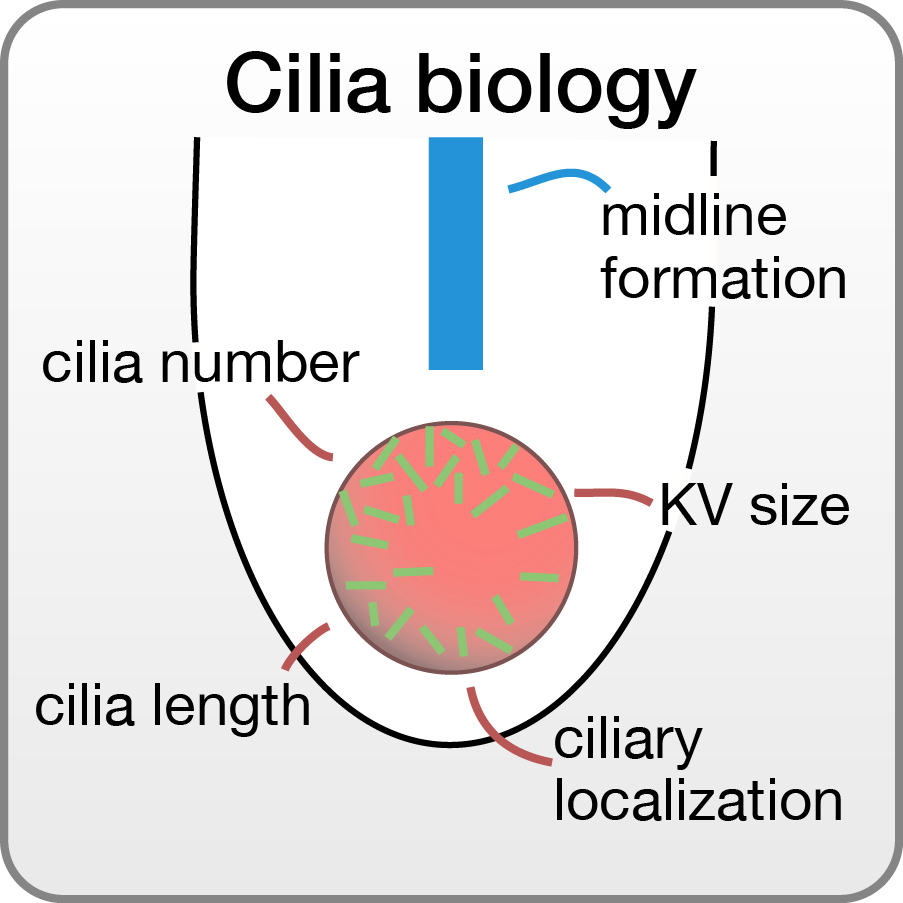
Cilia are microtubule-based protrusions from the surface of postmitotic cells that orchestrate a plethora of cellular signaling. Motile cilia are further capable of propelling body fluids and sensing fluid flows. When cells fail to form cilia or when cilia don’t function properly diverse afflictions can develop. Such ciliopathies include congenital malformations (i.e. impaired left-right asymmetry of internal organs), infertility, retinal degeneration or polycystic kidney disease. The clinical symptoms of cilia deficiencies also resemble those of centrosome deficiencies (i.e. microcephaly). We investigate cilia formation and function using zebrafish and cells, which we manipulate or which origin from patients with cilia defects.
We routinely evaluate genetic variants, which have been identified in patients, for their impact on protein expression and function. As model we use yet again the zebrafish as it offers testing of patient variants in an entire organism rather than an isolated cellular system. We cooperate with human geneticists and are always happy to offer such analyses to other labs. So far, we have analyzed variants of DVC1, a gene associated with progeria, and GRK5 variants, which we detected in patients with congenital heart defects and concomitant heterotaxy.
