26.07.2018- New study reveals structural basis of tetherin counteraction
HIV-1 Nefs are cargo-sensitive AP-1 trimerization switches in tetherin downregulation
Kyle L. Morris1, Cosmo Z. Buffalo1, Christina M. Stürzel2, Elena Heusinger2, Frank
Kirchhoff2, Xuefeng Ren1* and James H. Hurley1,3*
1Department of Molecular and Cell Biology and California Institute for Quantitative Biosciences, University of California, Berkeley, Berkeley, CA 94720, USA
2Institute of Molecular Virology, Ulm University Medical Center, 89081 Ulm, Germany
3Molecular Biophysics and Integrated Bioimaging Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA
A collaborative study between James H. Hurley's lab in Berkeley and researchers from the Institute of Molecular Virology in Ulm published in volume 174, issue 3 of Cell provides structural insights into how HIV-1 counteracts antiviral cellular factors.
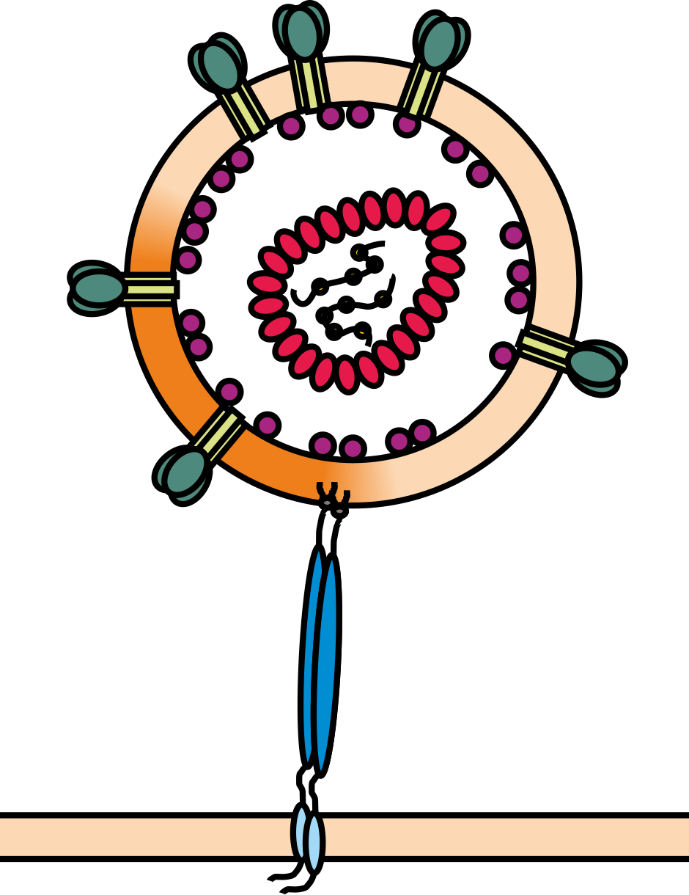
For efficient replication and transmission, human and simian immunodeficiency viruses (HIV and SIV) require so-called accessory proteins. One of them is the multi-functional factor Nef (Negative Factor) which downregulates various immune receptors (e.g. MHC-I and CD4) from the surface of infected host cells. Moreover, Nef acts as adaptor that counteracts antiviral cellular proteins, named host restriction factors, which are powerful weapons against viral pathogens. The membrane-anchored protein tetherin for example blocks the release of progeny virions from infected cells (see Figure 1) and is counteracted by the Nef proteins of most SIV and certain HIV strains.
The central findings of this study, which was supported by CRC1279, are based on a near-atomic reconstruction of the so-called closed trimer between Nef and its cellular targets (see Figure 2). Via cryo-electron microscopy, a multimeric complex formed by HIV-1 Nef, the cellular clathrin adaptor AP-1, the small GTPase Arf1 and tetherin has been determined at a resolution of 3.7 Å.
Together with their American colleagues, Frank Kirchhoff and his coworkers Christina M. Stürzel and Elena Heusinger showed how Nef regulates the formation of distinct complexes that downmodulate MHC-I or tetherin from the cell surface. Interestingly, tetherin is retained in the Golgi whereas Nef-mediated downmodulation of MHC-I receptor results in its lysosomal degradation. This study for the first time provides a structural explanation why Nef targets different proteins to different pathways: Only with tetherin, Nef forms the closed trimer while with MHC-I, an open trimer is observed that triggers clathrin-mediated transport. Moreover, the authors identified subtle structural differences in the Nef proteins of pandemic group M and epidemic group O HIV-1 strains that explain why only the latter efficiently antagonizes human tetherin. This resulted in a striking example of the enormous adaptability of HIV-1, likely contributing to its efficient spread: Since they cannot use their Nef protein, pandemic HIV-1 group M strains evolved the accessory protein Vpu as potent tetherin antagonist. So the lesson we can learn from HIV is: Take advantage of all your resources, stay flexible and carry on!