Combining tools to improve anti-cancer activity
First published: 14 June 2018, Advanced Science (https://doi.org/10.1002/advs.201701036)
Boosting Antitumor Drug Efficacy with Chemically Engineered Multidomain Proteins
Seah Ling Kuan, Stephan Fischer, Susanne Hafner, Tao Wang, Tatiana Syrovets, Weina Liu, Yu Tokura, David Yuen Wah Ng, Andreas Riegger, Christina Förtsch, Daniela Jäger, Thomas F. E. Barth, Thomas Simmet, Holger Barth, and Tanja Weil
Targeting and combating tumors in a more specific and efficient way is a central goal in modern cancer therapy. Current strategies involve e.g. the combination of classical chemotherapeutics with highly specific monoclonal antibodies. However, success is limited by side effects and resistance development. Within the framework of CRC 1279, researchers from Ulm (Medical Center and University) and Mainz (Max-Planck Institute of Polymer Research) jointly used the toolboxes of Pharmacology, Toxicology, Chemistry and Material Science in order to engineer a synthetic multidomain protein complex. The complex, termed SST3-Avi-C3, consists of two major components:
1) The chemically modified peptide hormone somatostatin (SST)
2) A mutant form of the C3 toxin from Clostridium botulinum
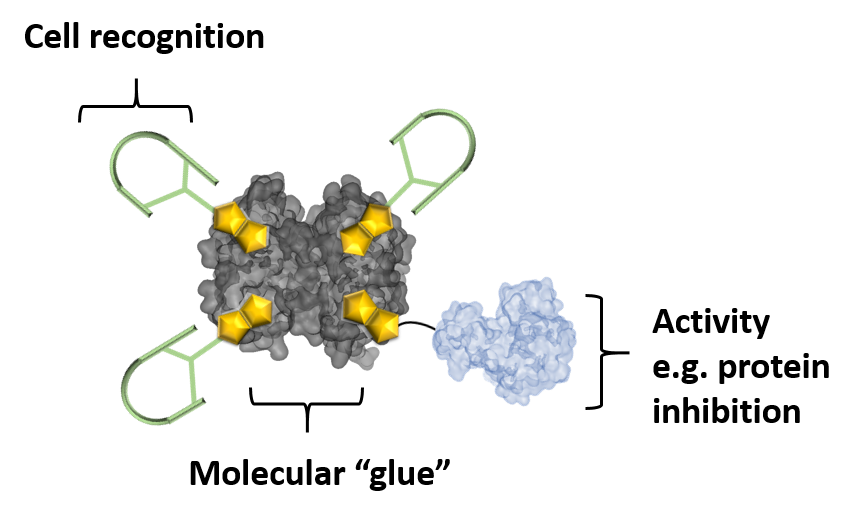
The strong binding of the tetrameric protein avidin (Avi) to biotin was employed as “molecular glue” to link the components. Since many tumors overexpress its receptor (SSTR2), SST was used to specifically target cancer cells. When three binding sites were occupied by biotinylated SST molecules (SST3-Avi), avidin was taken up into human lung cancer cells even more efficiently. To the fourth site, a biotinylated toxin enzyme C3 was attached. C3 inhibits Rho-mediated processes like endothelial cell migration and blood vessel formation and thus prevents tumor angiogenesis.
This study shows that the synthetic multiprotein complex SST3-Avi-C3 was selectively taken up by cancer cells, inhibiting Rho protein. Remarkably, the complex boosted antitumor activity of the conventional chemotherapeutic doxorubicin in human cancer cells and xenografts. Compared to the marketed antibody bevacizumab (Avastin), SST3-Avi-C3 reduced tumor growth with about ~100-fold higher efficiency. Moreover, although the formation of neutralizing antibodies has been observed in patients, resistance development does usually not deteriorate treatment efficiency of bacterial toxin-derived drugs.
Taken together, this work presents an innovative strategy to integrate a variety of bioactive entities and drug candidates into multidomain protein complexes. Such combinations expand the therapeutic repertoire and could help to optimize current cancer treatment. Moreover, by exploiting other specific cell-targeting peptides, this modular approach will yield further multidomain protein complexes for targeted drug delivery into cells and transport across the blood-brain-barrier.