Preventing infection without harming: New antiretroviral peptide discovered in human hemofiltrate
Members of the CRC 1279 identified a peptide that blocks HIV and SIV infection without interfering with the physiological function of the cellular entry cofactor.
Pandemic strains of HIV-1 are picky: usually they use only the chemokine receptors CCR5 and CXCR4 as cofactors for infection. Their relative HIV-2, that has infected about 2 million people, mainly in Sub-Saharan Africa, as well as simian immunodeficiency viruses (SIVs) are more promiscuous. They also utilize GPR15, a G protein-coupled receptor (GPCR) to enter their host cells.
GPCRs are involved in numerous cellular and pathological processes and represent highly attractive drug targets: Around 30% of all current drugs address GPCRs. However, molecules that block the pathological function of a GPCR usually also interfere with its physiological role. Not in this case: A research team led by Prof. Frank Kirchhoff from the Institute of Molecular Virology identified a peptide that blocks GPR15 without impairing its natural function in chemokine binding and cell signalling. The results have just been published in „PNAS" .
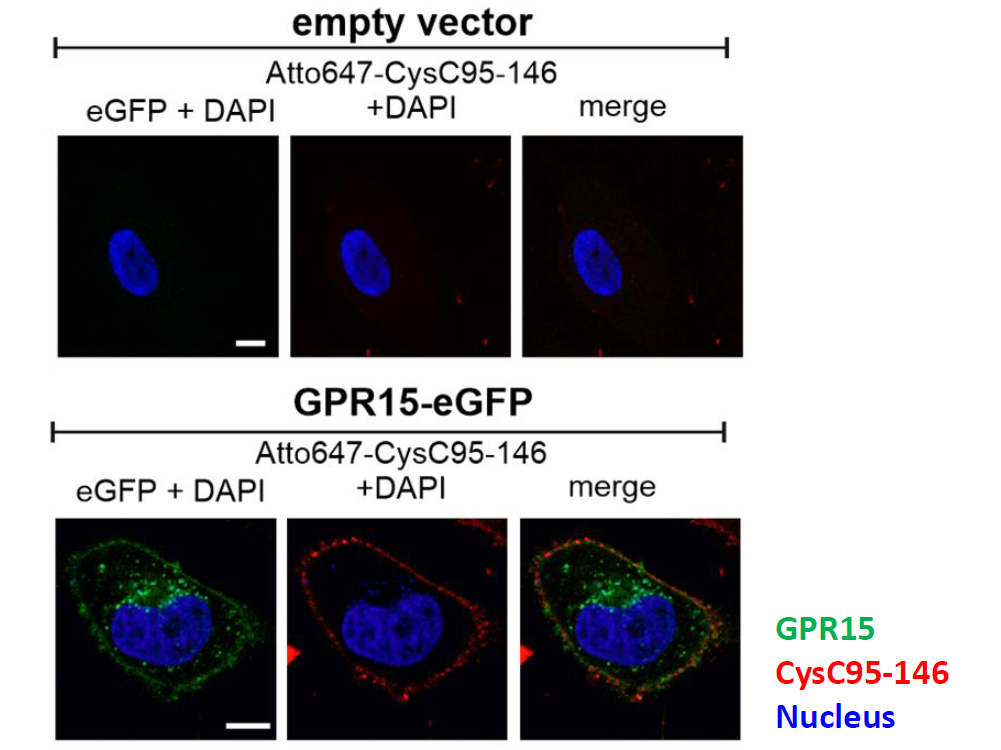
In order to find a molecule that blocks GPR15-mediated HIV-2 and SIV infection, the virologists screened a hemofiltrate-derived peptide library provided by Ludger Ständker from the Core Facility Functional Peptidomics at Ulm University Medical Center. This library, consisting of around 300 fractions contains essentially all peptides and small proteins that circulate in human blood. The fractions were added to cell cultures before they were exposed to different immunodeficiency viruses.
By the same procedure, teams around Kirchhoff and his colleague Prof. Jan Münch already identified endogenous peptides that bind to CCR5 or CXCR4 and thus potently prevent HIV-1 infection. Now, they discovered that C-terminal fragments of the protein cystatin C efficiently inhibit HIV-2 and SIV infection, both in cell lines and blood cells from human donors.
Interestingly, GPR15L, the chemokine ligand of GPR15 that was recently discovered by the group of Klaus Seuwen (Novartis, Basel) a collaborator of Kirchhoff’s team, did not inhibit lentiviral infection. „Our results suggest that GPR15L binds to another region of the receptor than the peptide that we identified“, says Manuel Hayn, PhD student in Kirchhoff’s lab and first author of the study. This might also explain why the Cystatin C-fragments do not interfere with the physiological function of GPR15. „These results serve as a proof of principle“, says senior author Kirchhoff. Maybe it is possible to identify or design such inhibitors for other GPCRs.
Moreover, Kirchhoff suspects that the generation of such antiviral peptides might represent a more general concept in innate immunity. The interdisciplinary research team showed that upon inflammation or viral infection, specific proteases become activated that generate the inhibitory fragments. „This could even be a driving force for the promiscuous coreceptor use of HIV-2 and SIV“, speculates Kirchhoff. In other words: The presence of peptides, that block a certain receptor could prompt a virus to become less picky and also use other cellular proteins as entry ports.