A05: Cellular and molecular responses to trauma-induced damage of the distal respiratory epithelium
PIs: H. Barth and M. Frick
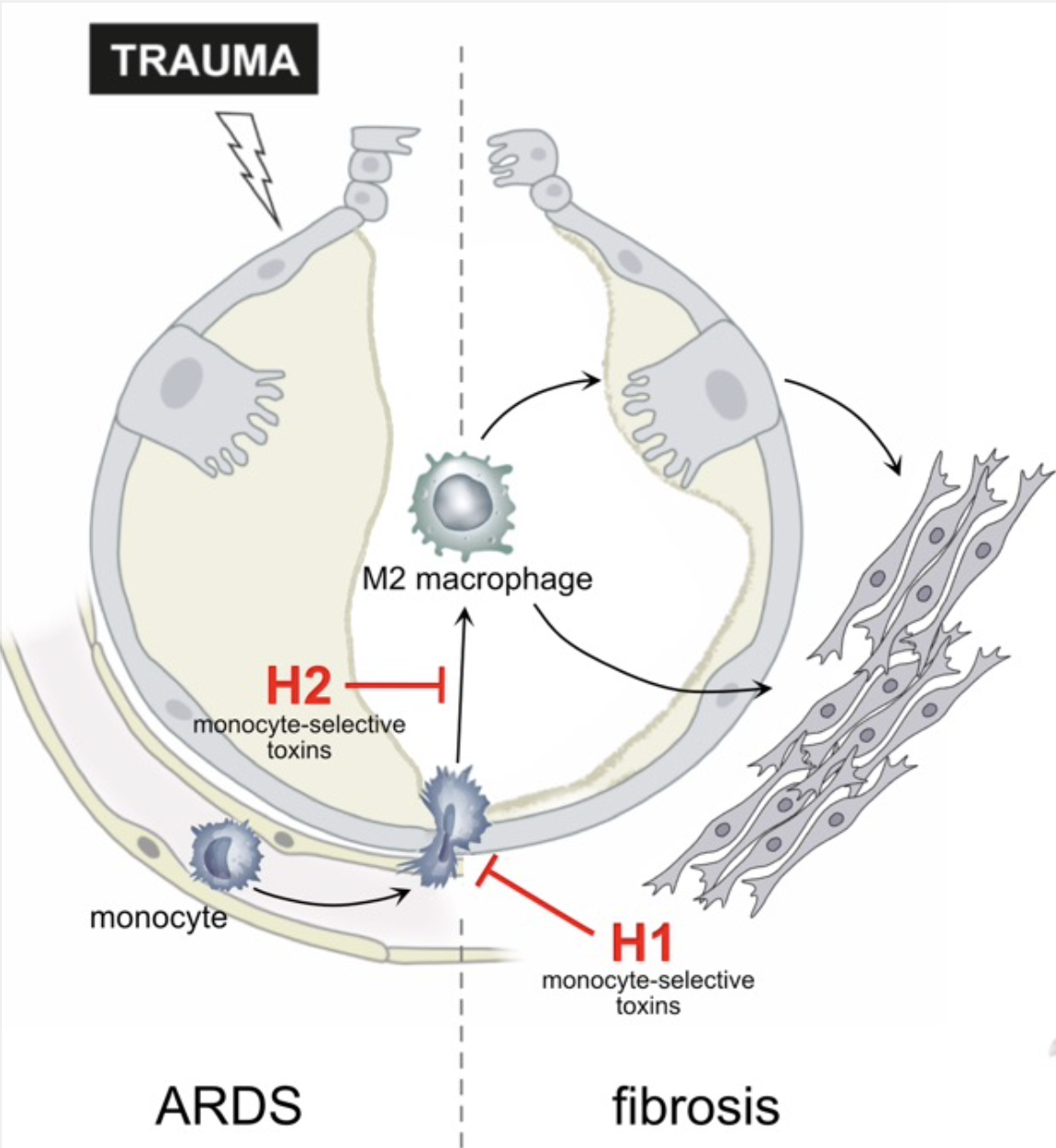
The acute respiratory distress syndrome (ARDS) is a common cause of respiratory failure in critically ill patients and frequently occurs in the setting of sepsis or severe trauma. ARDS is caused by disruption of the alveolar- capillary barrier separating air and blood in the lung. The resolution of ARDS requires repair of the damaged alveolar tissue to restore lung function. A substantial proportion of patients who survive the acute phase of ARDS, however, develop progressive and fatal pulmonary fibrosis. This is a result of impaired repair of lung injury in the fibroproliferative phase of ARDS. Immune cells, in particular monocytes recruited to the injured lung, play a crucial role in the resolution of lung injury. However, excessive recruitment of monocytes and polarization into M2 macrophages has been linked to fibrotic remodelling and development of pulmonary fibrosis. It has therefore been proposed that selective inhibition of monocyte recruitment to the injured lung and differentiation into M2 macrophages may relieve fibrosis severity. Yet, it is still unclear whether 1) inhibition of monocyte recruitment will adversely affect epithelial repair, 2) selective inhibition of monocyte differentiation into M2 macrophages limits pro-fibrotic signals in the lung, and 3) if M2 macrophages drive development of fibrosis directly or via activation of the epithelium. We will address these questions, based on extensive preliminary work and using our humanized in vitro models of the alveolar barrier that were established during the previous funding periods. We will inhibit monocyte/macrophage recruitment to the alveolus and their differentiation in these models using macrophage-selective, pharmacological inhibitors based on bacterial protein toxins. We will use previously developed inhibitors and generate improved versions using an established supra-molecular complex platform. These experiments will help elucidating whether targeted pharmacological inhibition of monocyte immigration and modulation of macrophage differentiation can ameliorate fibrotic remodelling during repair of the alveolus after trauma.
Projektleiter
Prof. Dr. Holger Barth
Institut für Pharmakologie und Toxikologie
Universitätsklinikum Ulm
Albert-Einstein-Allee 11
89081 Ulm
Tel: +49 731 500-65503
Fax: +49 731 500-65502
holger.barth(at)uni-ulm.de
Prof. Dr. Manfred Frick
Institut für Allgemeine Physiologie
Universität Ulm
Albert-Einstein-Allee 11
89081 Ulm
Tel.: +49 731 500 23115
Fax: +49 731 500 23242
manfred.frick(at)uni-ulm.de
Homepage