A06: Therapeutic targeting of small extracellular vesicle (sEV) release and uptake in post-traumatic hyper-inflammation
PIs: T. Seufferlein
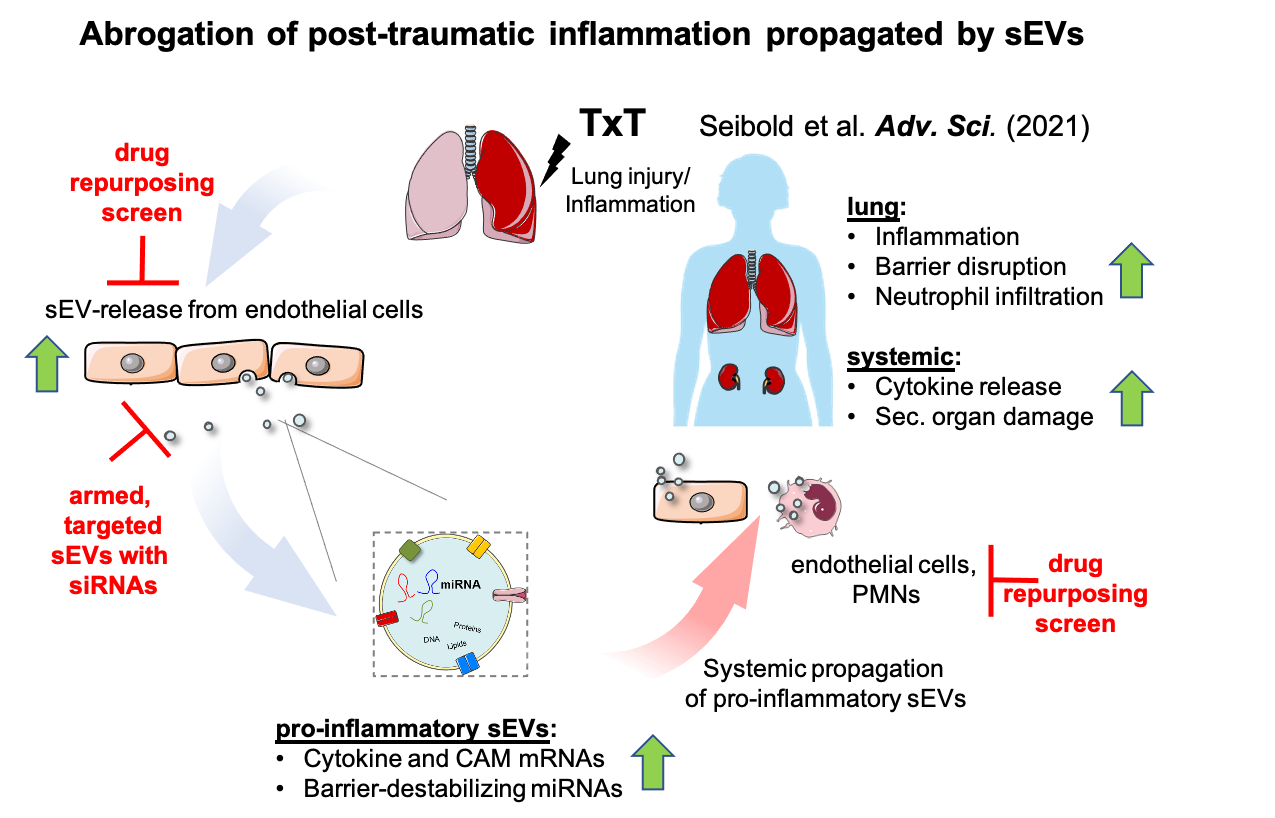
Major physical trauma triggers the release of damage- and pathogen-associated molecular patterns, cytokine expression, neutrophil-mediated inflammation, and physiological barrier disruption. If this trauma response turns unbalanced, systemic inflammation causes severe complications. We have previously demonstrated that increased release of small extracellular nanovesicles (sEVs, exosomes) from endothelial cells after thorax trauma (TxT) propagates local and systemic inflammation. Pharmacological inhibition of sEV-biogenesis in mice after TxT significantly normalized plasma-sEV concentrations, reversed adverse molecular signatures, impaired neutrophil infiltration, systemic inflammation, barrier destabilization and secondary acute kidney injury. These effects were mediated by systemic transfer of transcripts for endothelial cell adhesion molecules, such as ICAM-1 and cytokines, like IL6 or CXCL8 by sEVs to the endothelium. Moreover, enhanced sEV- release with similar cargos was also detectable in the plasma of polytrauma patients. Thus, we want to test the hypothesis that inhibiting sEV-secretion or -uptake by endothelial cells/neutrophils might be a promising strategy to attenuate aberrant inflammation after severe trauma. There are currently no clinically approved inhibitors available for a sensible use in patients. We therefore propose a drug repurposing screen to identify viable sEV-secretion and -uptake inhibitor candidates. Preliminary experiments have already optimized screening protocols. Besides, we will generate targeted, therapeutic sEVs with siRNAs against cytokines to impair sEV-loading of pro-inflammatory transcripts in ICAM-1-positive endothelial and lung alveolar cells. Here, also proof-of-concept experiments have been successfully conducted in vitro. Additional optimization and testing will be done in the 3D-lung model developed by A05 and drugs as well as therapeutic sEVs will be characterized in TxT mice together with A01 and Z02. Vice versa, A06 will provide sEV-expertise to other projects in the CRC (e.g. A01, A07, C05, C06 and A09N).
Projektleiter
Prof. Dr. Thomas Seufferlein
Zentrum für Innere Medizin
Klinik für Innere Medizin I
Albert-Einstein-Allee 23
89081 Ulm
Tel.: +49 731 500 44501
Fax: +49 731 500 44502
thomas.seufferlein(at)uniklinik-ulm.de
Homepage