B03: Oxytocin and H2S after traumatic brain injury and hemorrhage with pre-existing early life stress
PIs: T. Merz, P. Radermacher
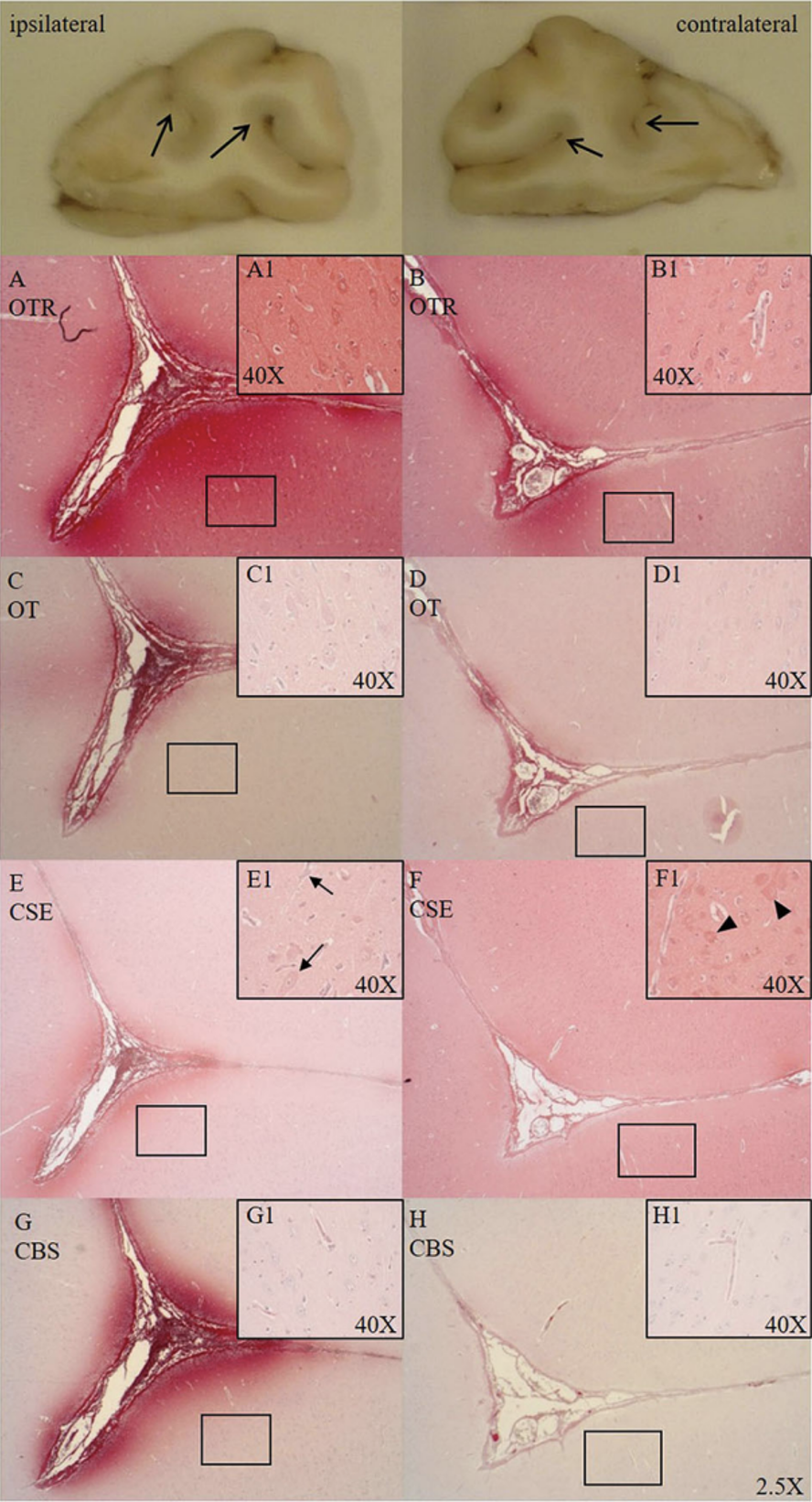
Traumatic brain injury (TBI) and hemorrhagic shock determine posttraumatic outcome, and thus "O2 debt repayment" and prevention of brain hypoxia are therapeutic cornerstones. However, restoring tissue perfusion represents an ischemia/reperfusion (I/R) injury due to formation of reactive oxygen (ROS) and nitrogen (RNS) species and subsequent systemic and neuroinflammation. "Early Life Stress (ELS)" worsens TBI both directly via enhanced neuroinflammation and indirectly due to increased incidence of cardiovascular disease during adulthood. ELS also down-regulates the oxytocin-receptor (OXT-R) and cystathionine-γ-lyase (CSE) expression, the major cardiovascular hydrogen sulfide (H2S) producing enzyme. The OXT/OXT-R and H2S/CSE systems interact both in psychological and physical trauma and in ELS-related cardiovascular disease. In the previous funding period, we showed that in the presence of cardiovascular disease, targeted hyperoxemia after combined acute subdural hematoma (ASDH)-induced TBI and hemorrhagic shock increased brain O2 tension, thereby attenuating neurological dysfunction and mortality. We also demonstrated that OXT-R and CSE interact after ASDH or hemorrhagic shock, and that H2S restored OXT-R expression after chest trauma and hemorrhagic shock. In this funding period, we will investigate the gender-specific role of ELS for brain injury and neurological function after combined ASDH-induced TBI and hemorrhagic shock. In a translational approach, the licensed drugs Syntocinon® and Natriumthiosulfat® will be used to assess the therapeutic impact of OXT and H2S. Readouts comprise neurological function, bi-hemispheric "multimodal brain monitoring", blood markers of brain damage and ROS/RNS, post mortem histology, immunohistochemistry, western blotting, mitochondrial activity and ROS/RNS release. In addition, neutrophil and PBMC metabolism and phagocytosis will be determined ex vivo to characterize immune cell function and metabolic switching upon trauma-induced activation.
Projektleiter
Dr. rer.nat. Tamara Merz
Universitätsklinikum Ulm
Klinik für Anästhesiologie
Albert-Einstein-Allee 23
89081 Ulm
Tel.: +49 731 500 60168
E-mail: tamara.merz(at)uni-ulm.de
Prof. Dr. med. Dr. h.c. Peter Radermacher
Universitätsklinikum Ulm
Institut für Anästhesiologische Pathophysiologie und Verfahrensentwicklung (APV)
Helmholtzstr. 8/1
89081 Ulm
Tel.: +49 731 500 60214
Fax: +49 731 500 60162
peter.radermacher(at)uni-ulm.de