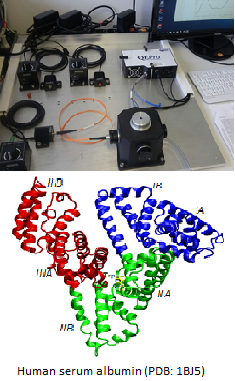
Overview
The folding equilibrium of the plasmaprotein HSA (Human Serum Albumin) is characterized from the fluorescence of a tryptophan residue at position 214.
Successive addition of the denaturant GdmCl leads to an increasing spectral shift of Trp-214 fluorescence, which allows to quantify the increasing fraction of unfolded protein.
The free energy change between folded and unfolded state is determined for two different pH-values by extrapolation to zero denaturant concentration.

![[Translate to english:] Button Overview Experiments](/fileadmin/website_uni_ulm/nawi.physik/dateien/data/biophyscis_lab/button_overview_experiments_350e.png)